The mitochondrion is one of the most important organelles in eukaryotic cells. Mitochondrial metabolism provides the energy required for the metabolism of the entire cell; meanwhile, they are involved in processes such as cell differentiation, cell communication, and cell apoptosis [58]. Most of SSB‐based bioprobes accumulate in mitochondria after passing through cytoplasm membrane may be because of an ~1.3 mV zeta‐potential of SSB derivatives [47] that makes it liable to be attracted by the ~−180 mV extreme negative potential of mitochondria. Modified by mitochondrial targeted groups, triphenylphosphonium (TPP) and pyridinium, Liu and coworkers developed SSB‐based probes 39and 40, which perform excellent mitochondrial imaging specifically [56, 59]. Moreover, such a modification often gives SSB fluorophores some additional attractive functions. For example, as illustrated in Figure 3.23b, as TPP is sensitive to negative charge, owing to the more negative mitochondrial membrane potential of cancer cells than normal cells, 39can selectively accumulate in cancer cell mitochondria and light up its fluorescence. Furthermore, this probe also shows high cytotoxicity toward HeLa cells as it could trigger mitochondrial dysfunctions. Thus, chemotherapy with 40is specifically targeted to mitochondria of cancer cells and monitored by itself. Images of HeLa cells costained with MitoTracker/LysoTracker shown in Figure 3.23c demonstrate that 39could accumulate on mitochondria with high efficiency, while for normal NIH‐3T3 cell lines, the mitochondria and lysosomes were all lit up without specificity. After incubating tetramethylrhodamine ethyl ester (TMRE), a mitochondrial membrane potential indicator, with 39pretreated HeLa cells, the fluorescence intensity of the cells decreased, indicating that 39can cause a reduction in the mitochondrial membrane potential. The reactive oxygen species (ROS) indicator, dihydroethidium (DHE), also clearly indicates that 39may cause ROS production and induce apoptosis. Further research showed that 39showed high cytotoxicity to HeLa cells even at low concentrations (0.5–1.0 μM). However, at a probe concentration of 8 μM, the viability of NIH‐3T3 cells was still higher than 80%. This treatment selectivity is related to the higher affinity of 39for cancerous mitochondria. Due to its great selectivity for imaging and eliminating carcinoma cells' mitochondria, 39was further self‐assembled together with the lysosome‐targeted photosensitizer AlPcSNa 4into NPs for mitochondria and lysosome‐targeted therapeutics, with synergistic chemo‐photodynamic therapy (PDT) functions associated with self‐monitoring by dual‐light‐up fluorescence [57]. With such fascinating characteristics, 39is expected to have promising applications in imaging‐guided precise cancer therapy. Additionally, 40was also found to have interesting property in differentiating brown adipose cells [59]. Other researches [60, 61] also found that 40could form a 1 : 1 host–guest complex specially with γ‐cyclodextrin (γ‐CD), which makes the fluorescence intensity on mitochondria in bioimaging enhanced about 30‐folds due to the restriction of intramolecular rotation of 40in γ‐CD. 40was also applied as fluorescence lighting‐up probe for the detection of protein aggregates.
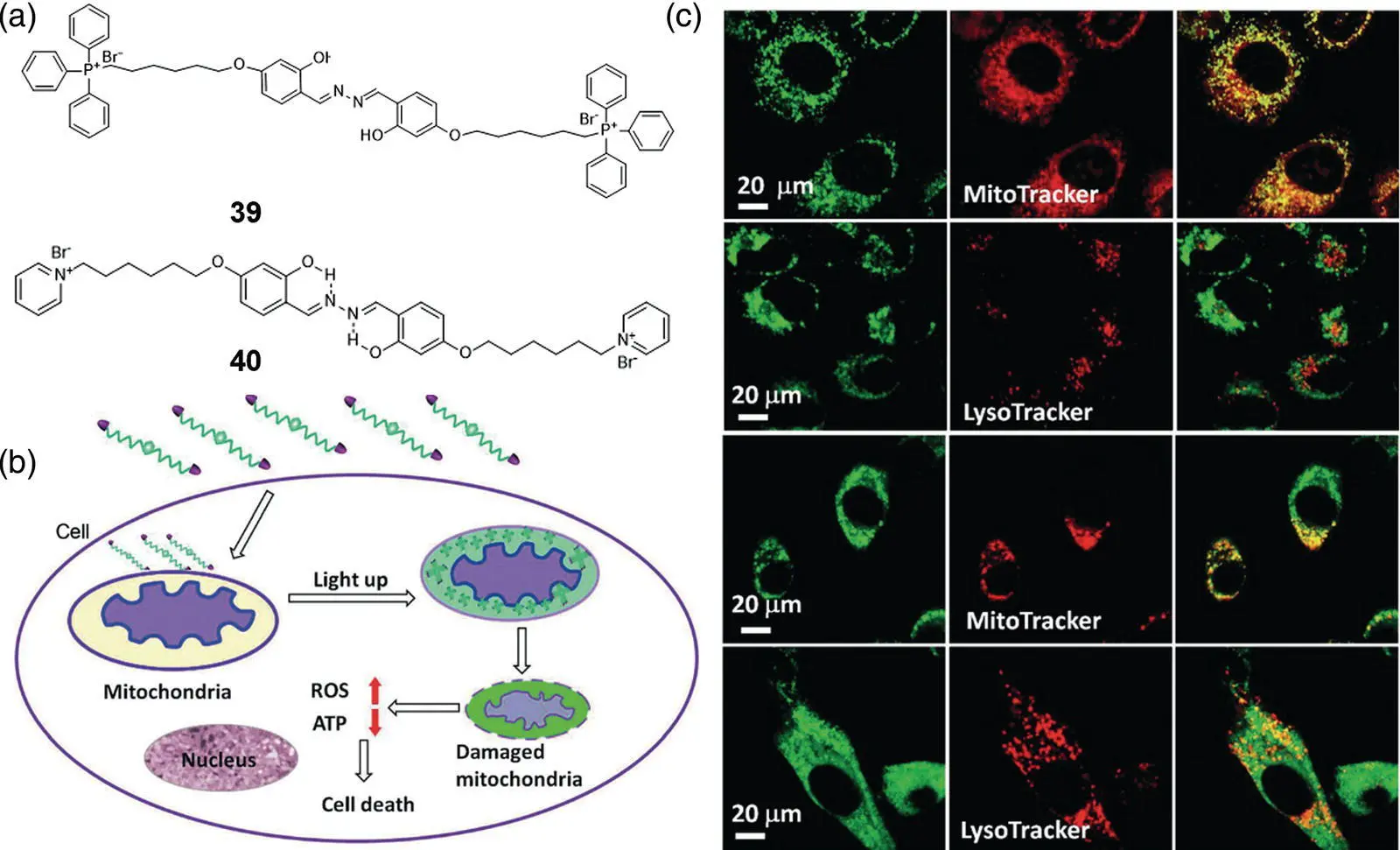
Figure 3.23 (a) Chemical structures of typical mitochondrial targeting SSB probes 39and 40. (b) Schematic representation of intracellular tracking and the therapeutic effect of 39in cancer cells. (c) Confocal microscopic images of HeLa cells and NIH‐3T3 cells after incubation with 39. The cells were costained with MitoTracker or LysoTracker.
Source: Reprinted from Ref. [56] (Copyright 2014 John Wiley and Sons).
By modifying with various targeting groups, a number of SSB‐based bioprobes for other cellular organelle‐specific imaging such as lysosome [62], cell membrane, and LDs were reported [63]. Besides, a series of SSB derivatives were also designed as photosensitizers for imaging and killing both gram‐positive and ‐negative bacteria over mammalian cells [64, 65].
Morpholine is widely used as a lysosome targeting ligand. SSB‐based bioprobes 41and 42thus show high affinity to lysosomes. By substituting hydroxyl groups of salicylaldehyde with acetyl groups, the ESIPT of 41was blocked and the fluorescence was quenched. After incubating with live cells, the probes accumulated in lysosomes and subsequently the protective acetyl groups were hydrolyzed by esterase, making them a specific fluorescent probe for lysosome esterase imaging [62]. A similar lysosome‐specific SSB bioprobe 42was developed according to the same principle and applied to monitor the autophagy process [66]. Figure 3.24B shows that the fluorescence of 42in HeLa cells colocalized finely with commercial lysosome dye with Pearson's coefficient as 0.9. Figure 3.24C shows that even if excess 42was removed before rapamycin treatment, the newly formed lysosomes were lit up. The results strengthen that the fusion of the autophagic region with the original lysosome occurred during autophagy, demonstrating the satisfactory application of 42in visualization of the lysosome and lysosome‐involved autophagy process.
LDs are subcellular organelles surrounded by a phospholipid monolayer and contain diverse neutral lipids such as triacylglycerol and cholesteryl esters. Some reports demonstrate that LD is a dynamically complex organelle involved in various physiological processes, and its metabolic balance and stability play a key role in living organisms. The abnormality of LD activities or numbers is a critical signal of various diseases, such as fatty liver diseases, type II diabetes, and inflammatory myopathy. Monitoring the location and distribution of LDs is therefore of great importance for early diagnosis of related diseases. Figure 3.24D displays two hydrophobic SSB compounds 43and 44applied in specific LD imaging for both live and fixed cells [67]. 43and 44emit yellow and orange fluorescence, respectively. Due to their ESIPT characteristics, the Stokes shift of 43and 44is as large as ~200 nm, which is superior to commercial BODIPY dyes for LD staining. As shown in Figure 3.24E, after incubation with 43or 44, the LDs in A549 cells were lit up with high resolution. The overlap rates of 43and 44with commercial LD dyes BODIPY were as high as 0.98 and 0.97, respectively, indicating their high LD‐targeting affinity. No significant inhibition of HeLa cell growth was observed in media with high concentrations of up to 10 μM 43and 44, indicating that these two probes have excellent biocompatibility.
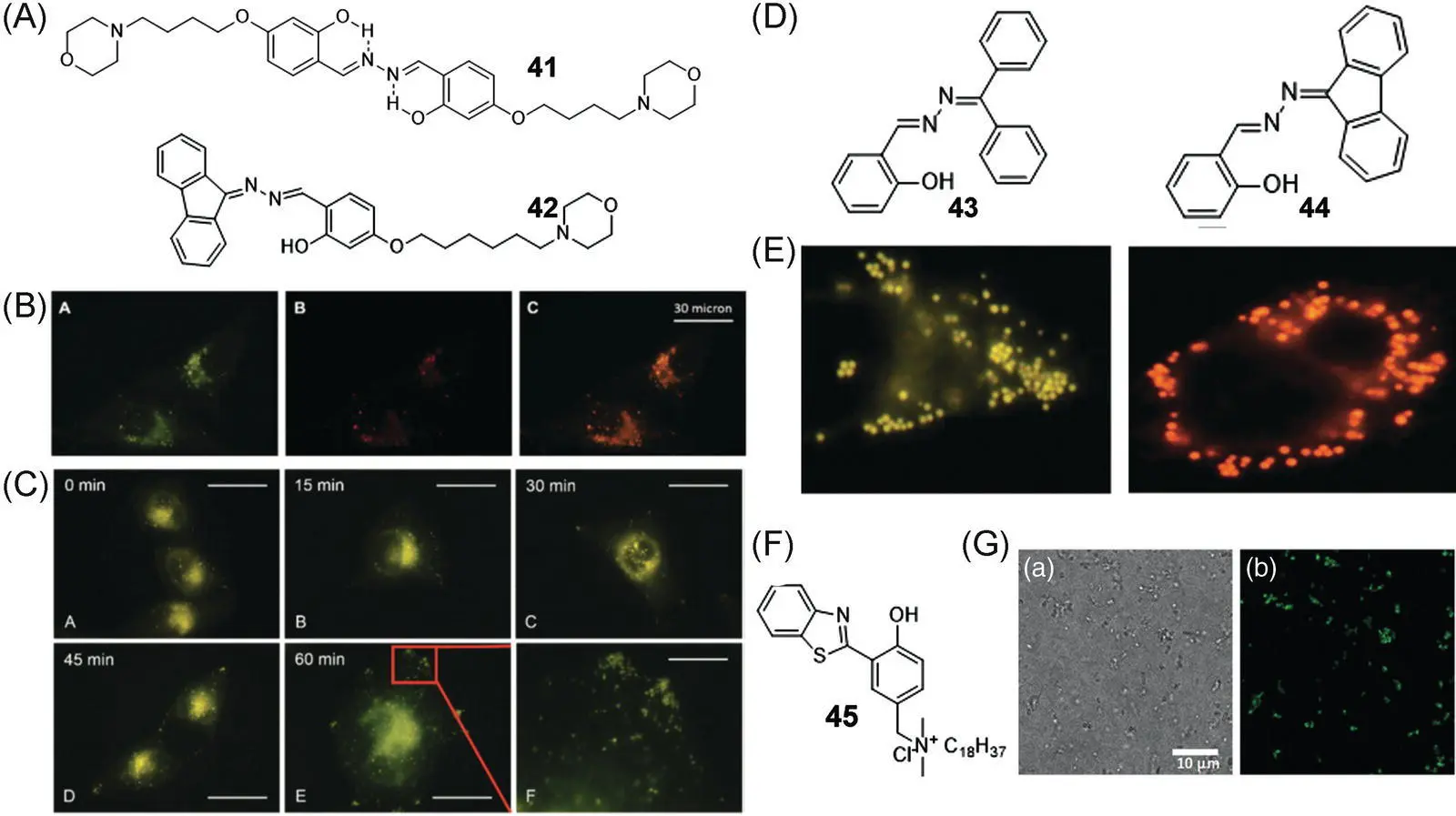
Figure 3.24 (A) Chemical structures of 41and 42. (B) Confocal images of HeLa cells stained with 10 μM 42and containing with 50 nM LysoTracker Red. (C) Fluorescence images of 42‐stained HeLa cells before and after rapamycin treatment for different periods of time.
Читать дальше














