Source: Reprinted from Ref. [75] (Copyright 2015 John Wiley and Sons).
By introducing rotary benzene rings into the symmetric positions of salicylaldehyde azine to increase the conformational flexibility thus achieving thermochromic switch provides a new idea for the design of stimulus‐responsive AIE materials. Based on this design principle, Tong's group modified 68with long alkyl chains and reported the single crystals of 69exhibiting three different fluorescence colors ( Figure 3.28e), which also showed reversible stimuli‐responsive fluorescence switching ( Figure 3.28d) [75]. X‐ray crystal structure analysis shows that the great differences existed in the molecular conformation and arrangement of the three crystals due to the presence of long alkyl chains, especially the small interplanar spacing of 69(Y) with intermolecular p–p interactions, which resulted in a red‐shift in emission wavelength ( Figure 3.28f). In addition, under the solvent fumigation of dichloromethane, the yellow form of 69(Y) changed to its green form 69(G) due to the molecular rearrangement from relatively close interplanar spacing and intermolecular p–p interaction therein to “monomer”‐like packing evidenced by DSC, polarized light microscopy, and PXRD. Annealing operations recovered an orange fluorescence from the green form with molecular arrangements similar to “dimers” ( Figure 3.28f). Such reversibly stimuli‐responsive characteristics of molecule 69were further applied to fluorescence printing and erasing in response to organic vapor and thermal stimuli ( Figure 3.28g).
Mechanical stimuli include pressure, stress, shear, friction, and pulverization; such stimuli‐responsive solid fluorescent materials have a wide range of applications in pressure sensing, memory devices, and optical recording due to their controllable fluorescent properties. Tong's group reported an SSB 70in 2011 ( Figure 3.29) [76]. When the crystal of 70was annealed by heating to 115 °C or by grinding, the solid fluorescence color changed from yellow to green with significant emission enhancement. In the structure of 70, a local dipole presents with N , N ‐diethylamino as the electron donor (D) and salicylaldehyde Schiff base structure as the electron acceptor (A). Dipole coupling of molecules between adjacent sheets stabilizes the crystal structure; the p–p interaction enhances the delocalization of excited state, revealed by single‐crystal X‐ray structural analysis, PXRD, and DSC. When the crystal is annealed or ground, the molecular arrangement changes and p–p interaction is therefore weakened resulting in the blue‐shift and enhancement of emission. Planarized fluorophores and push–pull electron groups play significant roles in the construction of solid materials with luminescence conversion properties.
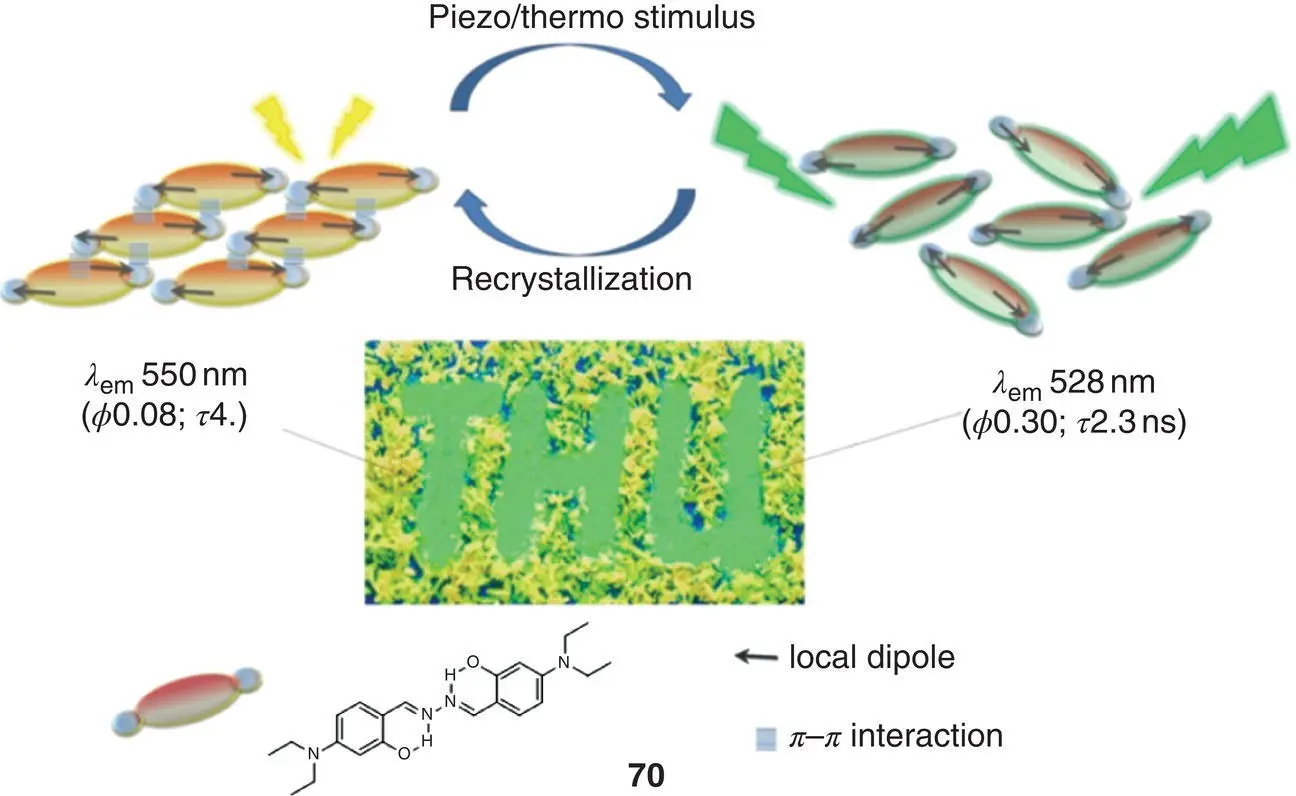
Figure 3.29 Schematic diagram of the molecular structure of 70and its mechanical/thermal stimulus response.
Source: Adapted with permission from Ref. [76] (Copyright 2011 American Chemical Society).
Laskar's group reported a reversible piezochromic molecules 71( Figure 3.30a) in 2016 [31]. The molecular arrangement of 71is J‐shaped in a solid or aggregate state, which reduces p–p interaction and promotes the ESIPT process with a strong yellow fluorescence emitting. After grinding 71solid, the intermolecular interaction weakens, resulting in a reversible blue‐shift of fluorescence from yellow to green, and returns to the state before grinding after a period of time ( Figure 3.30b, c). Another type of mechanoresponsive molecule 72( Figure 3.30d) was also reported by this group in 2017 that undergoes fluorescent discoloration under different external stimuli including shear force (grinding), axial pressure (hydraulic press), and temperature [21]. When a shearing force and an axial force are applied to 72, fluorescent color changes from blue to green. Particularly, the process is irreversible under the action of shearing force, while the molecule can slowly return to the initial state after axial force is applied. When 72is under low temperature in liquid nitrogen, the fluorescence color becomes blue‐green and the fluorescence gradually returns to blue after replacing at ambient conditions ( Figure 3.30e, f). Crystal structure analysis reveals that every two molecules form an antiparallel molecular pair via hydrogen bonding; the adjacent molecular pairs are then connected to form a chain, and the adjacent chains are then laterally connected to form a sheet structure. By comparing with the crystal data in liquid nitrogen, the molecular pair structure retained at low temperature, and there existed four kinds of intermolecular interactions when the molecular pair linked into a chain, accompanied by the electron distribution variation, which resulted in fluorescent color change ( Figure 3.30g).
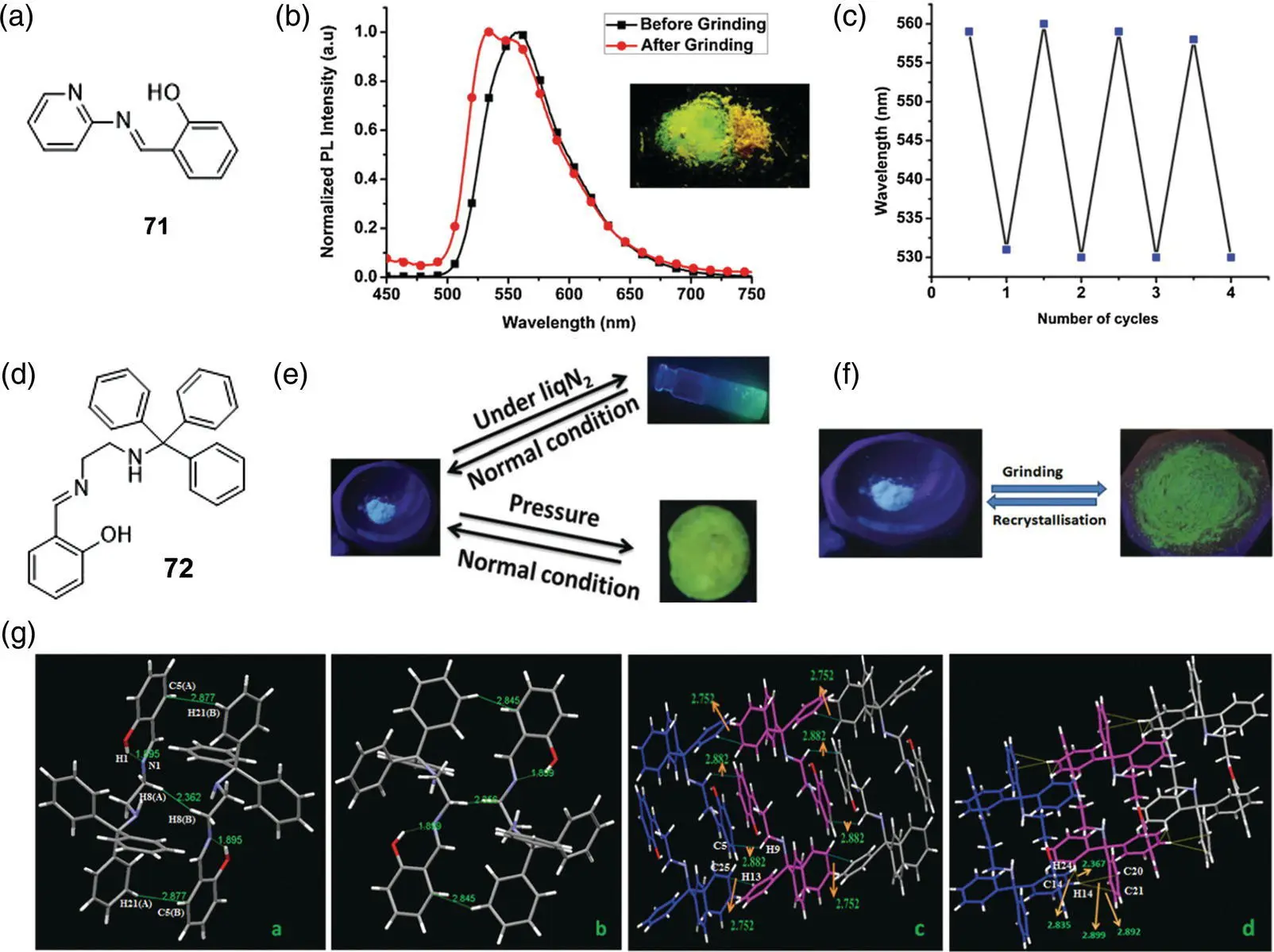
Figure 3.30 (a) Molecular structure of 71. (b) Normalized emission spectra of 71before and after grinding. (c) Switching of emission wavelength (∼553–530 nm and vice versa) after and before grinding, respectively.
Source: Reprinted from Ref. [31] (Copyright 2016 Elsevier B.V.).
(d) Molecular structure of 72. (e) Luminescence images of 72under various conditions ( λ ex= 365 nm). (f) Luminescence images of the as‐synthesized and ground samples of 72(photographs taken under a 365‐nm UV illumination). (g) (a and c) Packing diagrams of 72at room temperature, and (b and d) packing diagrams of 72at liquid N 2temperature (interactions shown in the figure are in Å).
Source: Reprinted from Ref. [21] (Copyright 2017 Royal Society of Chemistry).
SSB derivatives are also designed to form NPs, which will allow the fluorescent material much more stable with multifunctionalities applicable for material science, bioimaging, and PDT. Two common design principles for SSB NPs are generally used. One way is growing a transparent shell such as silica out of the SSB fluorogens' aggregate core. As Figure 3.31a and b shows, SSB fluorescent aggregates encapsulated in silica nanoparticles (SiNPs) were reported according to the Stober standard method [77]. Three SSB‐based AIEgens 73, 74, and photoresponsive compound 75were noncovalently embedded into SiNPs during the polymerization of silicate ester monomers to prepare AIE luminogen‐embedded fluorescent SiNPs (AIE‐FSNPs‐1–3). Compared with the conventional ACQ fluorophore fluorescein embedded in SiNPs prepared in the same way, AIE‐FSNPs exhibit an ~10‐fold fluorescent intensity; therefore, they show much higher sensitivity in further analytical application. Additionally, AIE‐FSNPs display satisfactory stability to external environmental variations. After experiencing multiple washings or under different pH buffers, the fluorescent spectra of AIE‐FSNPs show no obvious change. By covalently modifying AIE‐FSNPs with DNA aptamer AS1411, Apt‐AIE‐FSNPs were prepared and showed specific binding to nucleolin overexpressed on the surface of various cancer cells (MCF‐7, HeLa, etc.), thereby distinguishing cancer cells from normal cells in cell imaging. As shown in Figure 3.31c, Apt‐AIE‐FSNPs‐1 emitted a strong green fluorescence under a 405‐nm laser excitation after incubating with human breast cancer cells MCF‐7 but exhibited no obvious fluorescent signal with normal cells MCF‐10A, indicating their perfect performance in specific cancer cell recognition. More interestingly, as shown in Figure 3.31d, since the fluorescence of 75was caged by photolabile group o ‐nitrobenzyl, 75‐encapsulated Apt‐AIE‐FSNPs‐3 initially emitted no fluorescence incubated with MCF‐7. After irradiation with a 365‐nm UV light, the o ‐nitrobenzyl group was left to recover the orange fluorescent signal. Such photoactivatable characteristics give Apt‐AIE‐FSNPs‐3 unique advantages in the selective imaging of target cells at a specific location of interest by controlling the site of UV irradiation at the desired time.
Читать дальше














