Photochromic or photoactivatable molecules, which achieve color or fluorescence switching under specific light radiation, have great application potential in the fields of molecular switches, molecular logic gates, photocontrollable materials, anticounterfeiting, and photolithographic patterns. A series of wavelength‐selective photoactivatable multicolored SSB molecules are shown in Figure 3.27[72]. Under the irradiation of certain UV light, the substituted quenching group on the hydroxyl of SSB is removed, and the ESIPT and AIE of the molecule are restored ( Figure 3.27a). Different caging groups endow the selectivity of activation wavelength for the SSB fluorophores, and the different substituents on the benzene ring structure can adjust the fluorescence color ( Figure 3.27b). In particular, when the caging group itself is a fluorescence emissive 7‐methoxycoumarin, the molecule shows a blue fluorescence of coumarin before activation and emits a mixed color of coumarin and SSB fluorophore after light activation. Photocontrolled fluorochromism is thereby achieved. These photoactivatable SSB had also been successfully applied to photolithographic patterns ( Figure 3.27c).
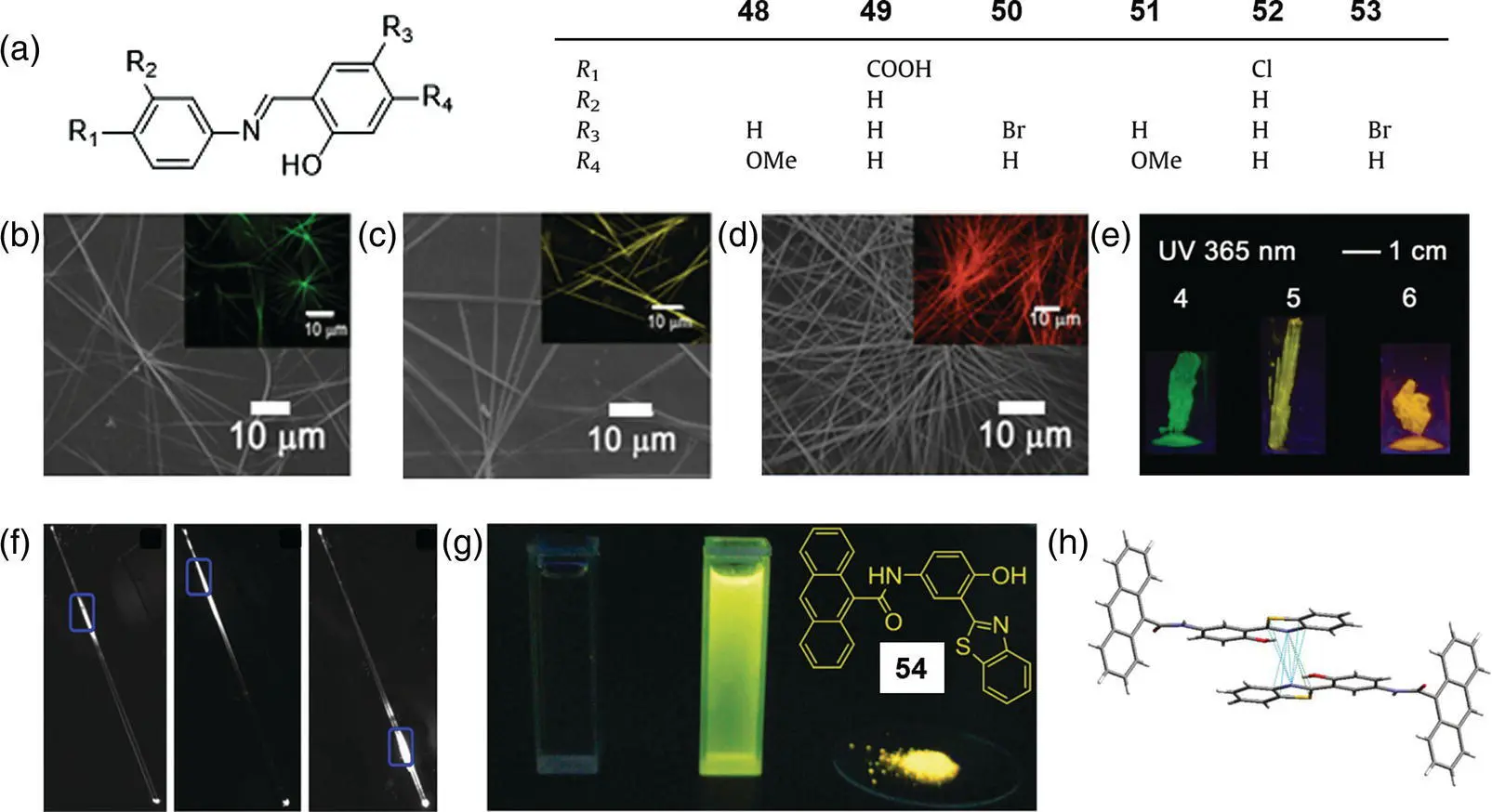
Figure 3.26 (a) Molecular structures of 48–53. (b–d) SEM images of 48–501D microrods generated by vacuum evaporating from ethyl acetate solution of corresponding compounds, respectively. Insertions were their fluorescence microscopy images. (e) Photograph of crystals for 51–53with centimeter size under a 365‐nm ultraviolet (UV) light. (f) Fluorescence microscopy images obtained by exciting identical microrods at three different positions without artificial staining.
Source: Reprinted from Ref. [70] (Copyright 2011 Elsevier B.V.).
(g) Photographs of 54under UV illumination at 365 nm in THF solution, water, and powder from left to right, respectively. Insertion was the molecular structure of 54. (h) N⋯ π interaction of 54.
Source: Reprinted from Ref. [71] (Copyright 2012 Royal Society of Chemistry).
The aforementioned photoactivation based on quenching groups is irreversible, while photoredox and photocyclization reactions are often used to design reversible photochromic molecules. However, the fatigue resistance of these photochromic systems is usually not desirable. Hou's group reported a class of reversible photochromic molecules 67that link SSB and tetraphenylethene (TPE) together with both AIE and ESIPT [73]. An SSB moiety can be converted from enol to keto under the irradiation of UV light to achieve the photochromic process ( Figure 3.27d). Molecule 67remained as yellow solid with an enol form, which had strong fluorescence at 545 nm and did not absorb at 550 nm. After UV light irradiation, 67turned red solid with a trans ‐keto structure, and the fluorescence intensity was significantly reduced while absorption at 550 nm is extremely enhanced. After removing the UV light, 67gradually returned to the previous state. Elevated temperature or visible light irradiation can promote the conversion rate of trans ‐ketone to cis ‐ketone, thereby increasing the rate of discoloration ( Figure 3.27f, g). Due to the reversibly photocontrollable luminescence of 67, it was applied in photopatterning materials with erasable properties ( Figure 3.27e).
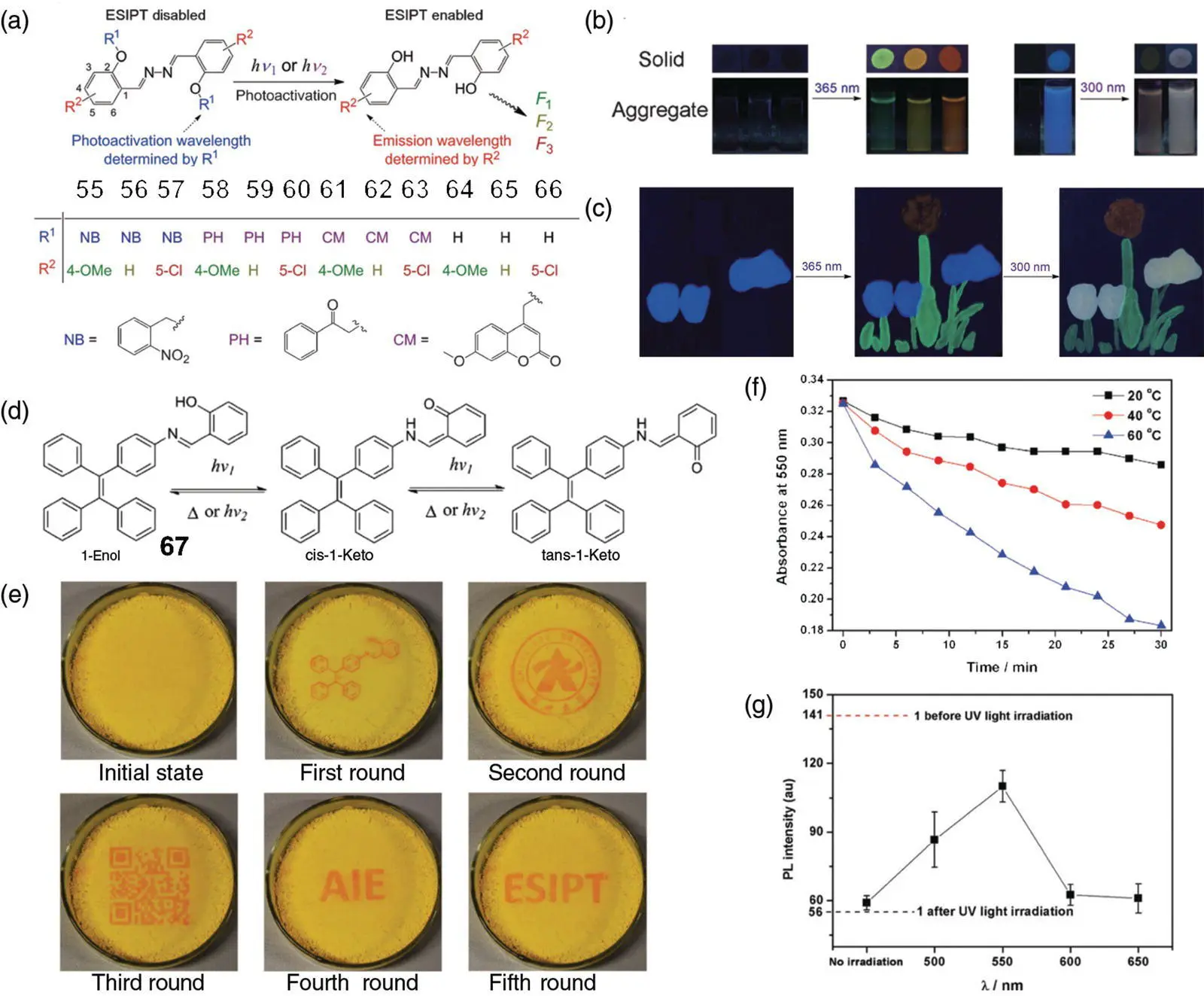
Figure 3.27 (a) Chemical structures of compounds 55–66and the scheme of photouncaging 56–64to yield 65–67, which are fluorescent at different wavelengths (colors) by UV irradiation at different wavelengths. (b) The multicolor fluorescence enhancement or change upon irradiation at 365 or 300 nm for 56(green), 57(yellow), 58(orange), 61(light orange), and 63(from blue to white purple) in their solid and aggregate (colloid solution) states. (c) Stepwise photoactivating a multiple‐color fluorescent image of flowers (blue, orange, and white purple) and leaves (green) made of 56, 58, and 63as solids by sequential UV irradiations at 365 and 300 nm.
Source: Reprinted from Ref. [72] (Copyright 2015 John Wiley and Sons).
(d) Proposed mechanism for the color change of 67upon UV irradiation. (e) Generating different patterns on the same film of 67. (f) The thermal fading kinetics of 67at different temperatures. (g) The dotted lines are the fluorescence intensity of 67at 545 nm before and after excess UV light irradiation. The scatterplot is the fluorescence intensity of UV‐irradiated 67at 545 nm exposed in light with different wavelengths for one minute.
Source: Reprinted with permission from Ref. [73] (Copyright 2017 Royal Society of Chemistry).
Upon external stimuli such as heat, force, solvent, temperature, etc., the arrangement of the material molecules can be varied among their polymorphisms. Therefore, the conformation, planarization, and intra/intermolecular interaction of the molecules change, which induces the fluorescence switching. Tong's group reported a class of reversible thermochromic SSB 68( Figure 3.28a) showing polymorph‐dependent AIE and ESIPT fluorescence [74]. Two fluorescent colors of 68single crystals 68‐Crys. (YG)and 68‐Crys. (G)were obtained from crystallization in concentrated ethyl acetate solution ( Figure 3.28b). The results of X‐ray crystal structure analysis showed that the dihedral angle between the benzene ring and the Schiff base plane in the two crystals was different, which led to the difference in molecular aggregates in the two crystals. In addition, according to differential scanning calorimetry (DSC) and powder X‐ray diffraction (PXRD), it is known that 68‐Crys. (G)undergoes phase transformation to the aggregate of 68‐Crys. (YG)by annealing at 231 °C, and 68‐Crys. (YG)converted to 68‐Crys. (G)by ablation treatment at 236 °C. During multiple annealing/ablation treatments, reversible switching of solid fluorescent color was obtained ( Figure 3.28c).
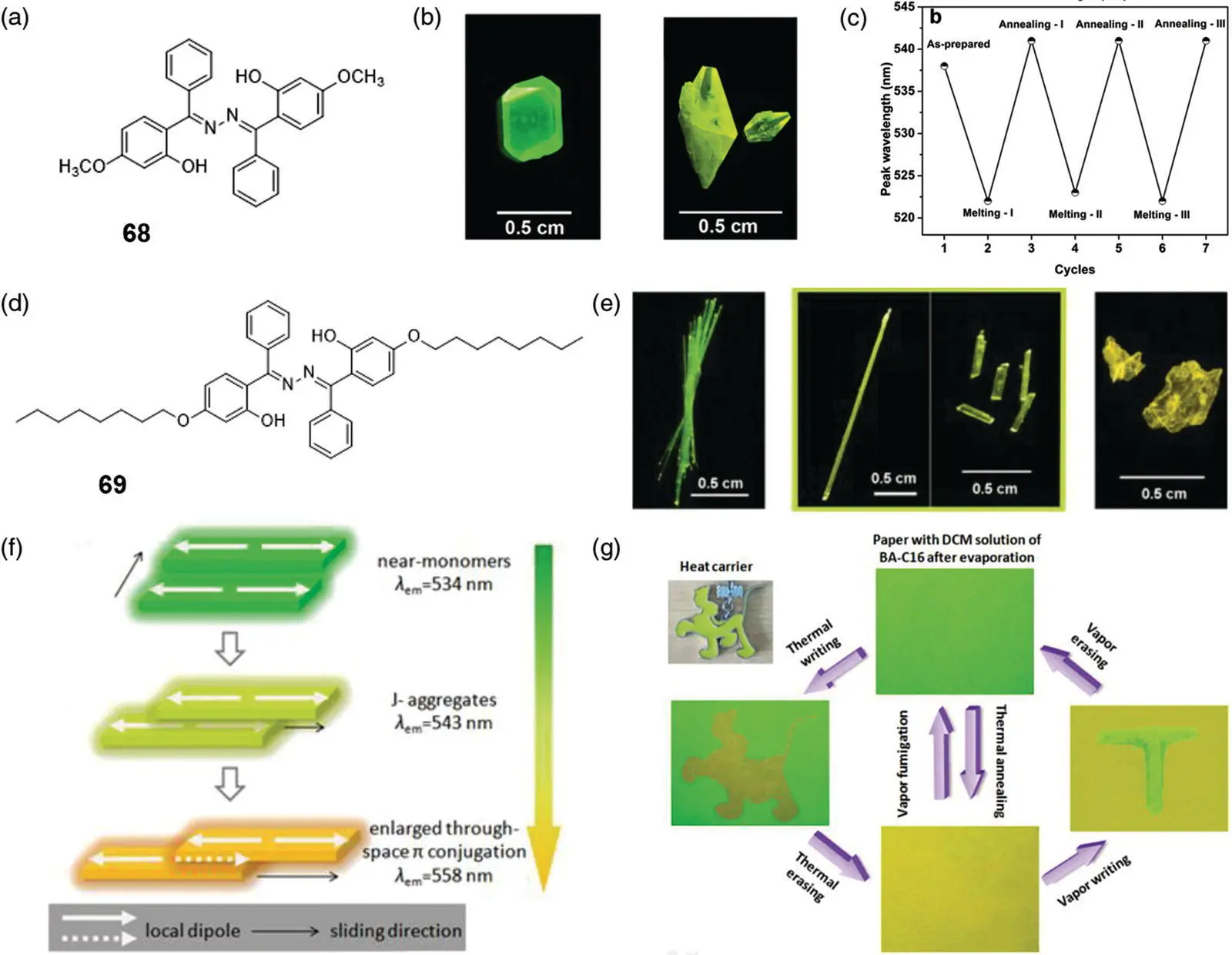
Figure 3.28 (a) Molecular structure of 68. (b) Polymorphic single crystals of 68( 68‐Crys. (G)and 68‐Crys. (YG)) under the illumination at 365 nm. (c) Peak position versus thermal treating cycle.
Source: Reprinted from Ref. [74] (Copyright 2013 American Chemical Society).
(d) Molecular structure of 69. (e) Photographs of polymorphous single crystals of 69( 69(G), 69 (YG), and 69(Y)). (f) Schematic illustration of the relationship between slip‐stacking modes and an emission wavelength of 69. (g) Reversible vapor‐ and thermo‐responsive fluorescence printing and erasing by using 69.
Читать дальше















