Diatom Microscopy
Здесь есть возможность читать онлайн «Diatom Microscopy» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Diatom Microscopy
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Diatom Microscopy: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Diatom Microscopy»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
The main goal of the book is to demonstrate the wide variety of microscopy methods being used to investigate natural and altered diatom structures. Diatom Microscopy
Diatom Microscopy — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Diatom Microscopy», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
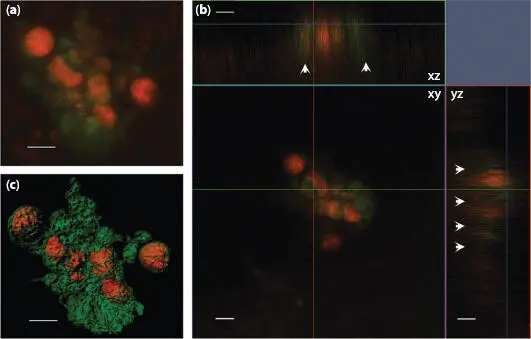
Figure 1.10 Laser confocal microscope images of H. hauckii-R . intracellularis symbiosis simultaneously excited by laser light at frequencies of 488 and 561: (a) z-stack image, (b) orthogonal views (xy, xz, yz) and (c) processed using the Contour Surface tool in IMARIS v.8.1 (Bitplane). White arrows indicate the fluorescence of the chloroplasts in xz and yz. Chloroplasts appear in green, and cyanobacteria trichome (filament) in orange. Scale bar = 5 μm. From [1.5] with permission of Oxford University Press.
Antifouling coatings can be fabricated by natural products that are dissolved in biofilms of bacteria and diatoms. A new method combining a high-throughput microplate reader, CLSM and nucleic acid staining has been developed to assess the activity of such coatings in terms of marine biofilm formation [1.60]. One approach to solving the problem of marine plastic marine debris (PMD) involves the use of microbes and biofilms. The combinatorial labelling and spectral imaging–fluorescence in situ hybridization (CLASI-FISH) tool makes it possible to visualize specific groups of microbes and elucidate their interactions with substrate surfaces, including microbial and diatom biofilms, which serve as polymer hydrolyzers capable of degrading PMD [1.6, 1.42, 1.62, 1.76]. Note that the marine ecological environment features numerous parasitic relationships. Vallet et al . [1.68] demonstrated that the flagellate marine oomycete (oomycete: commonly known as water mold, a kind of eukaryotic microorganism that is very similar to fungi. It is not organized with chlorophyll and thus does not perform photosynthesis. It requires nutrients after in vitro decomposition and then absorbs again) L. coscinodisci can infect Coscinodiscus granii diatoms. Figure 1.12presents the infection mechanism in which algal carbolines accumulate in the reproductive form of the parasite, as revealed by a single-cell analysis based on AP-MALDI-MS and CLSM.

Figure 1.11 Live cells, biosilica and biosilica-associated organic matrix from transformant strains expressing Sin1-GFP Nor Sin1-GFP C. The fusion proteins were expressed under control of the endogenous Sin1 promoter and terminator sequences. The ‘Live cell’ panels show confocal fluorescence images (z-projection) of individual cells in girdle view (left panel and third panel from the left) and in valve view (second panel from the left). Green color indicates the GFP fusion proteins and the red color is caused by chlorophyll autofluorescence. The biosilica and organic matrix panels show bright field microscopy images (BF) and the corresponding epifluorescence microscopy images (EF) of material isolated from Sin1-GFP N- or Sin1-GFP C-expressing transformants. Scale bars in all images = 2 μm. From [1.32] with permission of Springer Nature.
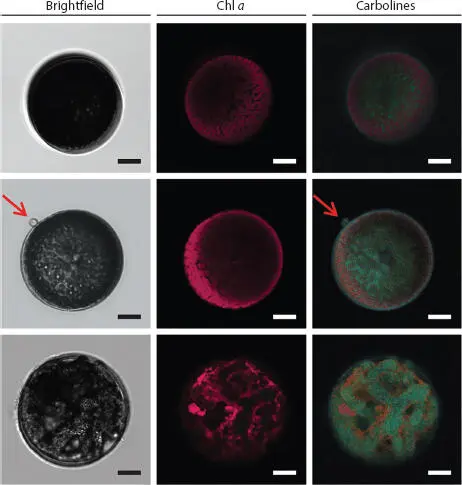
Figure 1.12 Quantification of carbolines in healthy and oomycete-infected diatom cells. The spatial localization and accumulation of carbolines was observed with CLSM in healthy (upper row), early infected (middle), and late infected (lower row) cells. These microscopic images were captured in bright field (left) and the autofluorescence emissions of chlorophyll a (Chl a) (middle) and carbolines (right) were recorded under an excitation wavelength of 405 nm. Scale bars = 10 μm. From [1.68] with permission of Springer Nature.
Diatoms are non-toxic organisms. In fact, diatoms have been used as a nutritious food source and in the treatment of cancer. Functionalized diatomite nanoparticles (NPs) have also been used as non-toxic nanocarriers for the transport of small interfering ribonucleic acid (siRNA) for the treatment of human epidermal cancer (H1355) and colorectal cancer [1.54]. CLSM revealed the cytoplasmatic localization of vectors and gene silencing by delivered siRNA in cancer cells incubated with siRNA-conjugated NPs (see Figure 1.13). Diatomite NPs coated with B12 (cyanocobalami) have also been used as a tumor targeting agent. The functionalization of this material was examined using various analytical techniques and the synthesis of the organometallic luciferin analogue of cyanoco-balami was detected by CLSM. This B12 modified diatom was shown to facilitate the targeted delivery of water insoluble inorganic complexes to tumors [1.11]. Diatoms can also be used in molecular biology to research organisms with complex plastids, which are suitable fluorescent proteins for the in vivo analysis of protein localization. CLSM has been used to measure GFP fluorescence emission at wavelengths from 500 to 520 nm, while P. Tricornutum plastid autofluorescence is measured at wavelengths above 625 nm. The fluorescent protein mRuby3 has been developed as a tag for in vivo studies on the localization of proteins. More importantly, CLSM is ideally suited to co-localization experiments using mRuby3, in which mRuby3 fusion protein and GFP-tagged proteins are expressed simultaneously [1.37].
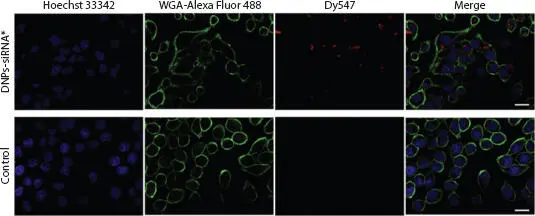
Figure 1.13 Evaluation of siRNA uptake and cellular internalization using confocal microscopy to estimate the ratio of red fluorescent cells (expressing Dy547) to total cells. The cells were imaged after being treated with siRNA*-modified diatomite nanovectors (first line) for 24 h with untreated cells as a control (second line). Cell nuclei and membranes were respectively stained with Hoechst 33342 and WGA-Alexa Fluor 488. siRNA was labeled with Dy547. Roughly 75% of the siRNA molecules in the first line are in H1355 cells, localized in cell cytoplasm rather than the nucleus. Scale bar = 20 μm. From [1.54] with permission of Elsevier.
The fact that folate receptors are highly expressed in the surface of many cancer cells means that folic acid can be used as a targeting ligand for the differentiation between normal and cancer cells. Rosenholm et al . [1.56] connected polyethylenimine (PEI) to diatom frustules. The attached amine group generated free electrons around the diatom frustule, which facilitates the connection to -N=C=S group, resulting in fluorescein isothiocyanate (FITC) fluorescence. The above was then connected to folic acid, FA, to form FITC/PEI/FA particles, which are visible under a fluorescence microscope. Because the internalization of the particle depends on the number of folate receptors, it was shown that 5-6 times more FITC/PEI/FA particles attach to HeLa cancer cells than normal cells (293), resulting in correspondingly strong green fluorescence, as shown in Figures 1.14a-1.14c. In addition, when mixing HeLa cancer with normal cells, the FITC/PEI/FA particles had a significant selective combination with cancer cells, showing these effects were particularly pronounced in cases HeLa cancer cells, as shown in Figure 1.14d.
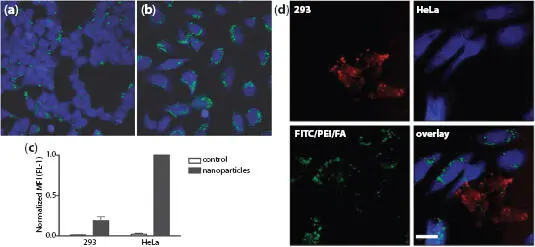
Figure 1.14 Specific particle endocytosis of FITC/PEI/FA-functionalized silica nanoparticles. Normal cells (a) or HeLa cells (b) were left untreated or incubated with nanoparticles (10 g/ml) for 4 h, after which extracellular fluorescence was quenched using trypan blue. The endocytosed particles with FITC label (green) inside CMAC-labeled (blue) cells were detected via confocal microscopy (a and b) or flow cytometry (c). Mean fluorescence intensity (MFI) values of FITC were normalized to particle endocytosis in HeLa cells. (d) Specific endocytosis of FITC/PEI/FA-functionalized silica nanoparticles in co-culture of HeLa cells (labeled using blue CMAC) and 293 normal cells (labeled using CellTracker Red). Scale bar = 30 μm. The results are representative of two independent experiments detected using a confocal microscope. From [1.56] with permission of American Chemical Society.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Diatom Microscopy»
Представляем Вашему вниманию похожие книги на «Diatom Microscopy» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Diatom Microscopy» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












