Diatom Microscopy
Здесь есть возможность читать онлайн «Diatom Microscopy» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Diatom Microscopy
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Diatom Microscopy: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Diatom Microscopy»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
The main goal of the book is to demonstrate the wide variety of microscopy methods being used to investigate natural and altered diatom structures. Diatom Microscopy
Diatom Microscopy — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Diatom Microscopy», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Confocal laser scanning microscopy (CLSM) involves the focusing of a low-energy laser source on a small spot (to prevent photo-bleaching) and the placement of a pinhole in front of the photodetector (to block out-of-focal plane signals) [1.40, 1.48]. In addition to enhancing the contrast and resolution of images, this approach enables the observation of samples at specific depths layer-by-layer (i.e., optical sectioning) as well as the reconstruction of images (i.e., layer stacking) to render the sample in 3D. CLSM can be used to observe cell samples of <50 μm; however, it is inapplicable to samples of >100 μm, due to limited light penetration resulting from the absorption and scattering of light at visible wavelengths. Note that applying light of higher intensity to compensate for a loss of photons by the confocal pinhole and imaging biological samples for a long time with visible wavelengths can greatly exacerbate phototoxicity and/or photobleaching.
Since the advancement of ultrafast laser technology, the shortcomings of CLSM have largely been resolved by equipping laser scanning microscopes with ultrafast lasers. This method (referred to as multiphoton microscopy) [1.3, 1.12, 1.38] typically uses near-infrared (NIR) excitation to minimize the water absorption in the tissue and thereby increases penetration depth to hundreds of micrometers. Importantly, the multiphoton effect only occurs at the focal plane (i.e., inherent optical sectioning) without the need for an additional pinhole to filter the out-of-focal plane scattering and the problem of emitted photon loss shown in CLSM. In addition, the use of a laser source with high peak power and low average intensity allows imaging over extended durations without inducing phototoxicity or photobleaching. Based on these advantages, multiphoton microscopy can be used for the 3D imaging of thick biological tissues in vivo or ex vivo . In the last decade, super-resolution optical microscopy [1.2, 1.26, 1.41] honored by the Nobel Prize in Chemistry 2014 is devised to overcome the diffraction barrier of light [1.28, 1.47], providing a greatly improved resolution on several tens of nanometer scale (i.e., an order of magnitude improvement over conventional optical microscopy). In addition, it is demonstrated in the far-field at arbitrary biological states and no need to pretreat the sample prior to investigation, which are the advantages over electron microscopy. Associated techniques mainly rely on fluorescence microscopy and advanced fluorescent probe [1.19, 1.66] with a fast on/off switching rate and better stability. It promotes the progression of single-molecule detection and cell and molecular biology in visualizing the fine structures and dynamic processes of single molecules inside cells or tissues.
In the following sections, we provide a comprehensive understanding of the application of the above-mentioned microscopy methods to the study of diatoms in this chapter. First, we discuss light microscopy in Section 1.2, which is not associated with fluorescence. It forms the image of diatoms using basic light characteristics such as scattering, diffraction and interference. Then, we discuss fluorescence microscopy and confocal microscopy in Section 1.3and Section 1.4respectively, which can provide images with high contrast, specificity and selectivity using fluorescent probes or the probes synthetized with diatoms. Next, we discuss multiphoton microscopy in Section 1.5, which enables high-contrast, label-free and deep imaging, as a patch up to the weakness of fluorescence-based microscopy. At last, we discuss the recent advanced imaging technique, super-resolution optical microscopy, in Section 1.6for the study of diatoms.
1.2 Light Microscopy
1.2.1 Phase Contrast Microscopy
The sensitivity of diatoms to variations in aqueous growth conditions (e.g., temperature, salinity, oxygen content, and pH) makes them excellent indicators of changes in water ecosystems (e.g., eutrophication, pollution and acidification) [1.15, 1.53]. Figure 1.1taken from phase contrast microscopy illustrates the diversity of diatoms in Baryczka stream (Poland), which were used as an indicator of water quality [1.44]. Besides, phase contrast microscopy has been used to study Azpeitia africana (a typical warm water diatom species) as an indicator of water temperature in the Pacific Ocean [1.71]. Diatoms are microscopic algae enclosed between two valves of hydrated amorphous silica. As shown in Figure 1.2, these intricate structures present a symmetric pattern of pores at the micrometer- and nanometer-scale. The highly symmetrical 3D geometry of the silica skeletons produces peculiar optical characteristics, similar to those produced by photonic crystals [1.14, 1.21]. This similarity has provided an interesting point of view in optical properties for light guiding and optical transducing in the electromagnetic field [1.8].
Quantitative phase imaging (QPI), as an extended form of phase contrast microscopy, makes it possible to measure the complex transmission function (i.e., the discrete Fourier transform (DFT) of sample under Rheinberg illumination, which contains amplitude and phase information) of diatom samples. Color contrast can be applied to images by introducing optical staining via an amplitude filter placed in the illumination path. Figure 1.3presents quantitative phase images of diatoms obtained via digital holographic microscopy [1.17]. It was obtained by Rheinberg filter simulation on the back focal plane of a microscope condenser lens positioned for Kohler illumination that occurs in the discrete Fourier transform (DFT) domain. Note that the nine point sources at the focal plane of the condenser, as shown in Figure 1.3c, are transformed to plane-wave illumination on the sample. Then, the resulting image is formed by the sum of the nine independent image intensities, of which each image corresponds to the spatial filtering to different regions of the DFT of the complex transmittance function, as shown in Figure 1.3e, which is done prior to an inverse DFT for returning it back to the spatial domain as an image. Furthermore, a similar approach was reported that a contrast was produced in image intensity, which is correlated with the phase delay introduced by the specimen. This method allows for a real-time imaging of cellular morphology with nanometric axial resolution. Figure 1.4shows two diatom cells recorded by QPI, in which the color filters, as a type of optical staining that controls light amplitude at specific wavelengths, placed in the illumination path are used to provide color contrast to images of phase objects. They are color-coded according to small values of local spatial frequency/ray angle, which make it possible to visualize the details of diatoms [1.18]. These results clearly demonstrate the applicability of QPI for the label-free color staining of biological samples.
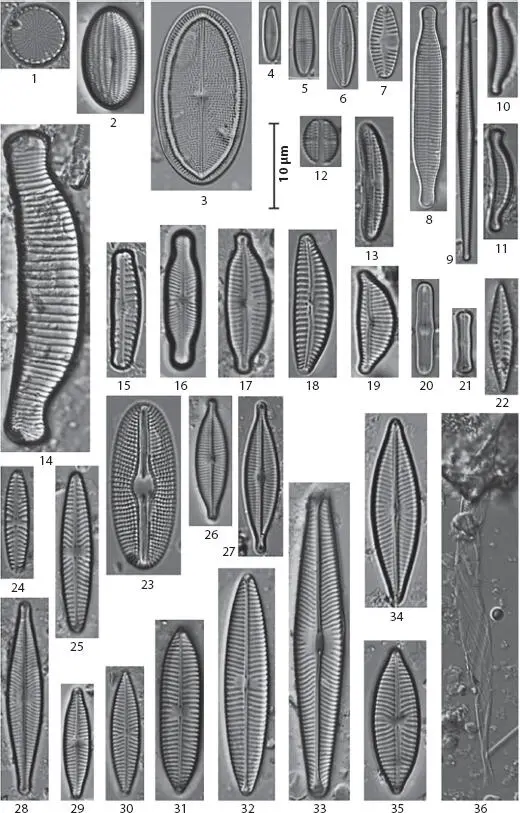
Figure 1.1 Selected diatoms taxa in the Baryczka stream obtained using phase contrast microscopy. Note that these images were used as an indicator of water quality. From [1.44] with permission of Bentus.
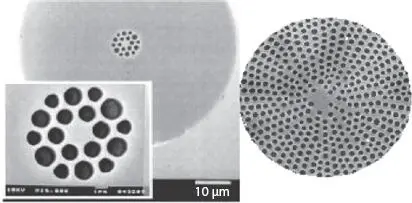
Figure 1.2 Comparison of diatom defects and man-made photonic crystal fiber. The circular holes and hexagonal lattice of the diatom is similar to the structure of a hexagonal lattice solid-core photonic bandgap fiber used to confine light via band gap effects. From [1.8] with permission of Elsevier.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Diatom Microscopy»
Представляем Вашему вниманию похожие книги на «Diatom Microscopy» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Diatom Microscopy» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












