The various reflex pathways with their respective final motor neurons throughout the brainstem and spinal cord are controlled for voluntary movement by neurons in motor centers in the brain which are collectively referred to as central motor neurons. Figure 1.3depicts generic central motor pathways. Corticospinal motor pathways with neuronal cell bodies in the cerebral cortex (A) are very important in primates but do not appear to be very important in initiating voluntary limb and body movement in domestic animals. Quite massive lesions destroying the cerebrocortical motor centers that would be devastating in higher species do not cause permanent demonstrable abnormality in the gait of large animals. After the acute effects of such large lesions in our veterinary species have resolved, there can be subtle deficits in motor functions such as jumping fences and hopping on the thoracic limb opposite the side of the lesion. In contrast, quite small lesions in the midbrain and medulla oblongata usually result in hemiparesis or tetraparesis because of damage to central motor centers and tracts such as the rubrospinal ( Figure 1.3B), reticulospinal ( Figure 1.3C), and vestibulospinal ( Figure 1.3D) pathways. These central motor neuronal pathways tend to have a calming effect on reflexes, particularly those involved with supporting the body against gravity such as the patella reflex. Because of this, central motor pathway lesions do not suppress reflexes and can indeed result in hyperactive reflexes and responses. The major role of these central motor pathways however is to direct the various final motor neurons in (voluntary) movement such that central motor pathway lesions will result in poor or absent voluntary effort (paresis or paralysis) in the face of very active spinal (and cranial) reflexes.
Just as brain motor centers help control the final motor neurons within the reflex arcs, the sensory inputs to these arcs are relayed to brain centers to give feedback on position sense (proprioception) touch, and pain perception (nociception) ( Figure 1.4). Sensory pathways travel to the thalamus, and probably to the somatosensory cerebral cortex, for the perception of pain. These pathways are multisynaptic and contain small fibers, both characteristics making them resistant to interruption. Proprioceptive pathways that are presumed to be consciously perceived (position sense at rest) travel to thalamic and thence to cerebral conscious proprioceptive centers. Other subconscious proprioceptive information (movement sense) is contained in spinocerebellar tracts that pass directly to the cerebellum.
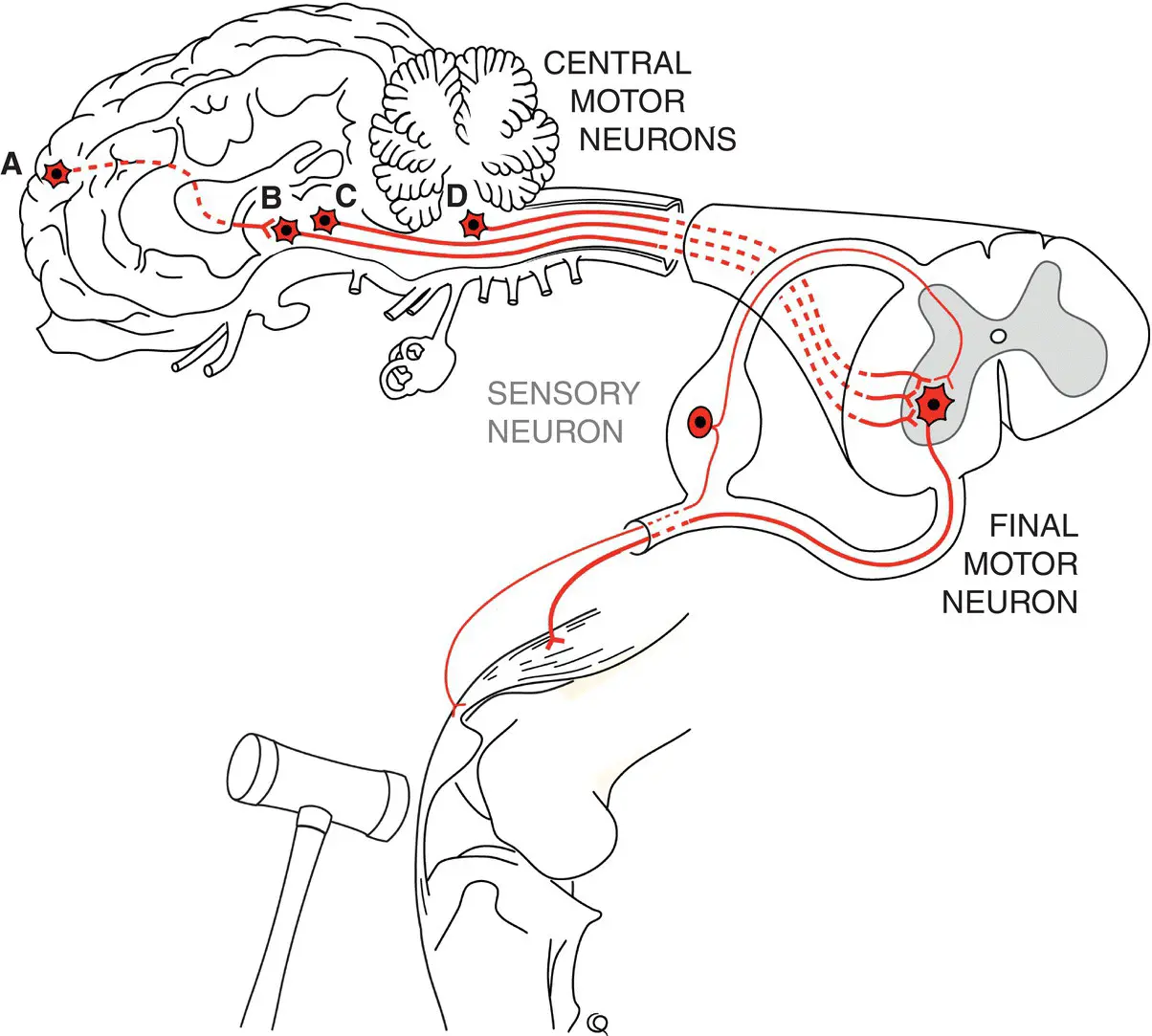
Figure 1.3 Central motor neuronal pathways predominantly originate in the brainstem and synapse on final motor neurons to effect motor activity. Depicted here are A the cortico (rubro)spinal, B the rubrospinal, C the reticulospinal, and D the vestibulospinal neurons and tracts.
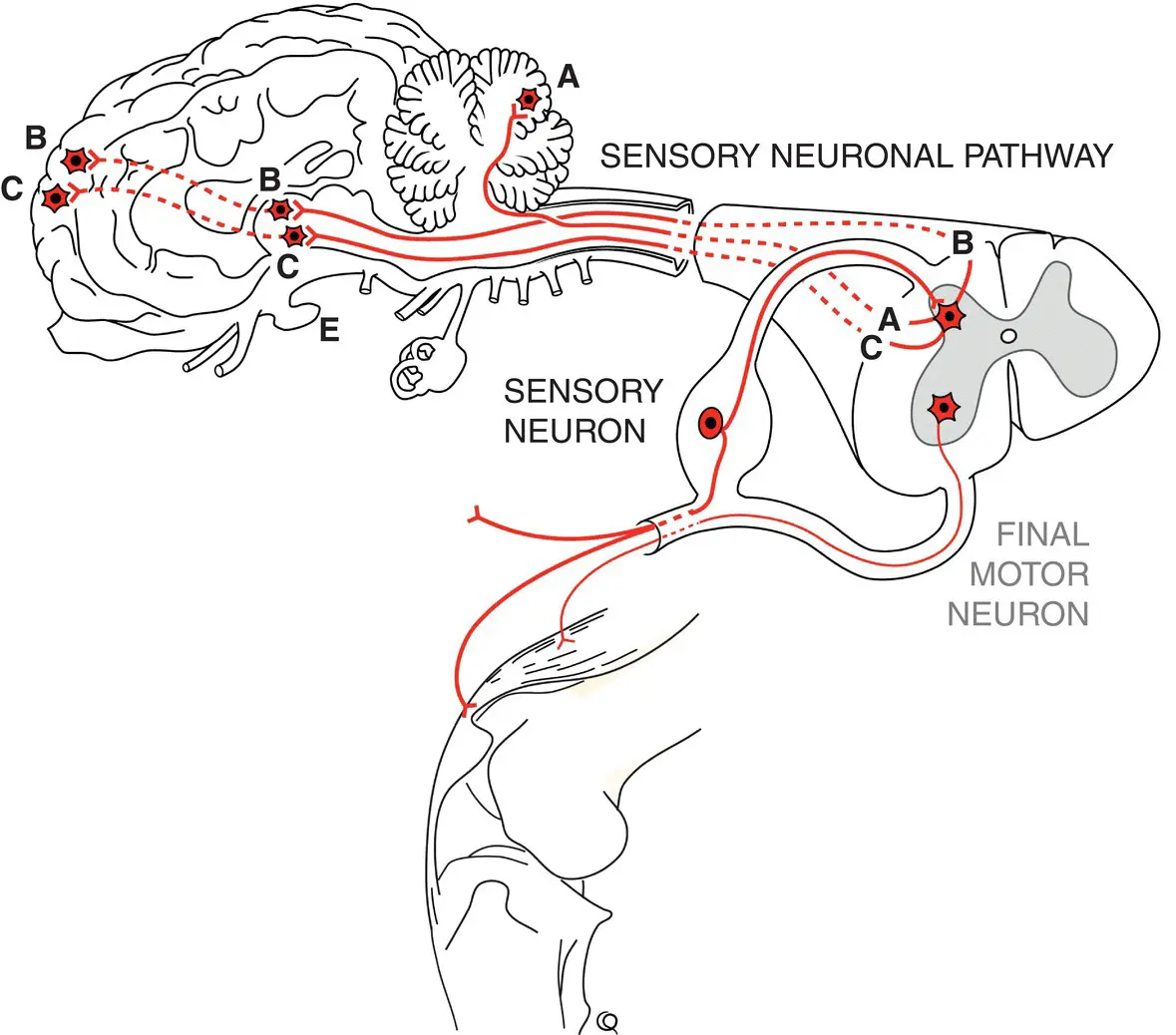
Figure 1.4 Sensory pathways convey somatic, proprioceptive, and visceral input to brain centers. Subconscious proprioceptive neurons A project to the cerebellum, whereas conscious proprioceptive neurons B and somatic and visceral afferent neurons C project to the thalamus and ultimately project to forebrain centers.
In summary, damage to the spinal cord at the level of a reflex arc at its final motor neuron will denervate the effector muscle, resulting in flaccid (loss of tone) paralysis with degrees of hypotonia and hyporeflexia at the level of the lesion. In contrast, a similar severe lesion cranial or rostral to a reflex arc will result in hypertonic (spastic) paralysis. The latter is characterized by loss of voluntary motor function as with a final motor neuron lesion, but the reflex is not diminished. Indeed, the reflex may be released from the calming influence of its controlling central motor pathways and be hyperactive with concomitant hypertonia (spasticity— sic .) or stiffness in body parts such as limbs. When a central motor pathway lesion causes a degree of spastic paralysis, the involvement of adjacent proprioceptive and nociceptive sensory pathways also results in ataxia and possibly hypalgesia, respectively.
Certainly, the concept of considering motor activity on the basis of “upper” and “lower” motor neuron function and lesions, even for quadrupeds, is entrenched in veterinary neurology, although not without some comment. 3,5Anatomically, upper and lower are not acceptable (with the exception of some cranial structures such as eyelids) as terms of direction in veterinary anatomy ( Nomina Anatomica Veterinaria, 6th Edition), 1making the use of these descriptors confusing to say the least. Also, in contrast to “upper” and “lower” motor function generators and pathways, the concept of central pattern generators has its merits when considering gait and posture in humans and animals. 11In this model, there is a generator of highly developed skilled motor activity initiated, in primates, in the cerebrum. A further generator of motor function, most prominent in our large animals, resides in the midbrain and rostral medulla oblongata. Finally, there is a generator at the spinal level that can initiate reflex actions and indeed probably is responsible for primitive gait patterns (viz. “spinal walking”); the interested reader may find further reading on this concept quite intriguing. 12–15For several reasons, therefore, there is a strong argument in veterinary anatomy and neurology to dispense with upper motor neuron (UMN) and lower motor neuron (LMN) terminologies: a strategy we have accepted for this text. The CNS pathways involved with initiating motor function and maintaining appropriate muscle tone and hence body posture are referred to as central motor pathwaysthat directly influence the final motor neuronsinnervating muscle to effect all motor functions. Many final motor neuron somata reside in the brainstem and spinal cord of the CNS with axons in the PNS, the exceptions being the presynaptic final motor neurons of the autonomic nervous system (ANS) which are wholly in the periphery.
The cerebellum is the major coordinating center for voluntary movement ( Figure 1.5). It synthesizes impulses received from the cerebral cortex, the brainstem motor centers, and spinocerebellar and vestibular (special) proprioceptive pathways. It then provides feedback impulses to the motor centers to coordinate all motor functions.
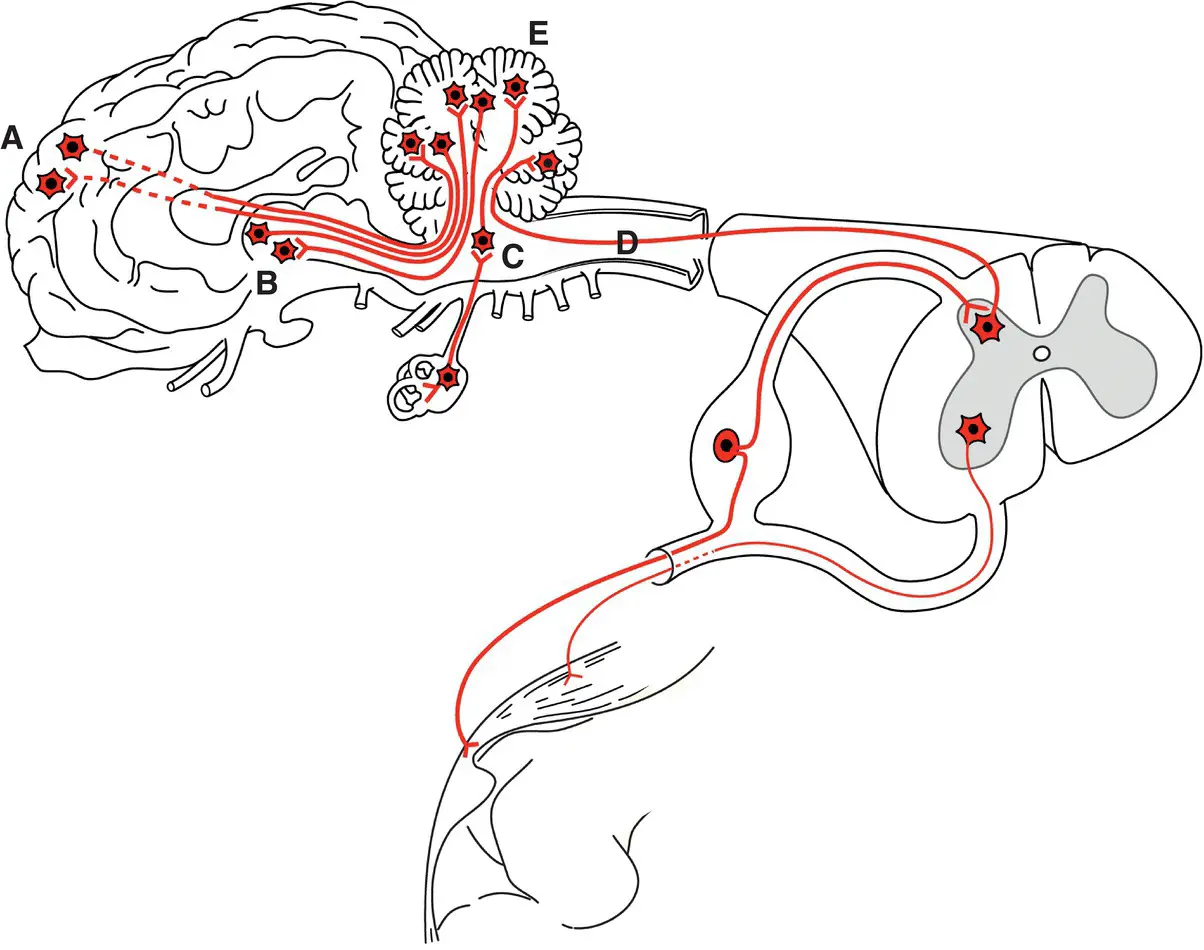
Figure 1.5 Important cerebellar connections include subconscious general proprioceptive input from the body such as via spinocerebellar pathways shown as D (same as A in Figure 1.4). Other input is received from cerebrocortical A and brainstem B motor areas and vestibular centers C. All cerebellar efferent output is from cerebellar cortical (Purkinje) neurons connecting to brainstem B and cerebrocortical A motor areas.
Cranial nerves (CNs), numbering I through XII, leave the forebrain and brainstem ( Figure 1.6). Some are involved with specialized modalities such as smell (CN I), sight (CN II), and balance and hearing (CN VIII). Some innervate eyeball muscles (CNs III, IV, and VI), muscles of facial expression (CN VII), pharynx and larynx (CNs IX and X), and tongue (CN XII). Others are involved with reflex functions such as facial reflexes (CNs V and VII) and the gag reflex (CNs IX and X). These cranial final motor neurons have central motor pathways controlling them. A final motor neuron lesion at the level of a CN or its nucleus can paralyze the motor function of the CN. Alternatively, a lesion affecting the efferent central motor pathways that influence the final motor neuron can curtail such CN motor function. As with spinal reflexes, in the latter case the structure ( e.g ., face) shows variable hypertonic, spastic paralysis ( e.g ., grimacing), with intact or hyperactive reflexes.
Читать дальше















