A more recently discovered initiation pathway that also responds to initial infection, called the lectin pathway, involves multimeric proteins called mannose-binding lectins (MBL), which are members of a family of PAMP-recognizing proteins called collectins. Collectins are calcium-dependent lectins (i.e., proteins that bind specifically to certain sugar residues in the presence of calcium). The MBLs bind mannose groups that are commonly found on the surfaces of bacteria but not on human cells. Recently, another group of PAMP-binding lectins, called ficolins (FCNs), which bind specifically to N-acetylglucosamine (GlcNAc) groups, have been shown to activate the lectin pathway in a similar manner. Collectins are produced by the liver and are part of what is called the acute phase response (or acute inflammatory response) to an infection—the initial onslaught by a variety of proteins, including cytokines and iron-binding proteins, that make it difficult for bacteria to multiply. Collectins that are bound to the surface of a bacterium not only sequester the bacteria into clumps that are then eliminated from the body by phagocytic cells, but they can also activate the complement cascade to form the C3-convertase via the lectin pathway.
Finally, antibodies generated through the adaptive defense system (to be discussed in more detail in chapter 4) can also activate complement by binding to the surface of bacteria and interacting with the C1-complex. Antibodies are blood proteins produced by B cells that bind to specific molecules on the bacterial surface called antigens. Antigen-bound antibodies can bind the C1 complex to activate it, initiating the cascade that ultimately leads to formation of the C3-convertase. Thus, both the innate and adaptive defense systems can trigger the complement cascade—yet another example of a link between the innate and adaptive defenses.
Before examining each of the pathways for complement activation in detail, it is helpful to understand the roles of the activated proteins produced by the series of proteolytic cleavages that comprise the cascade. Regardless of how the complement pathway is activated, the same key activated components are produced: C3a, C3b, C5a, and C5b ( Figure 3-11).
C3a and C5a are proinflammatory molecules that stimulate granulocytes, such as basophils and mast cells, to release the vasoactive substances in their granules, thereby increasing the permeability of blood vessels and facilitating the movement of phagocytes from blood vessels into tissue ( Figure 3-2). C5a also acts as a potent chemokine, signaling phagocytes to leave the bloodstream and guiding them to the infection site. Once PMNs or monocytes have left the bloodstream, they move along a gradient of C5a to find the locus of infection.
At the site of infection, C3b binds to the surface of the invading bacterium, enhancing the ability of phagocytes to ingest (engulf) the bacterium. This process of marking phagocyte targets is called opsonization, and any molecule that does this marking is referred to as an opsonin. As will be seen in the next chapter, antibodies can also perform this function. Without opsonization, phagocytes have difficulty ingesting a bacterium unless it is trapped in a small space. Because bacteria do not stick well to the phagocyte surface, the action of pseudopod encirclement can actually propel the bacterium away from the phagocytes—analogous to the way a fish can slip from your hands as you try to grab it. Phagocytes have C3b receptors on their surfaces that specifically bind C3b ( Figure 3-12). Thus, by binding to the bacterial surface, complement component C3b gives the phagocyte something to grab on to as it tries to engulf the bacteria. Antibodies bound to bacterial surfaces can also act as opsonins, because a conserved portion of the heavy chain of the antibody, the Fc region, is recognized by Fc receptors on the phagocyte. The difference between these two processes is that antibodies bind to specific molecules on the surface of a bacterium, whereas C3b binds nonspecifically to glycosyl groups commonly found on bacterial surfaces. The combined effect of C3b and Fc receptors binding to their respective ligands on the bacterial surface works synergistically to enhance phagocytosis.
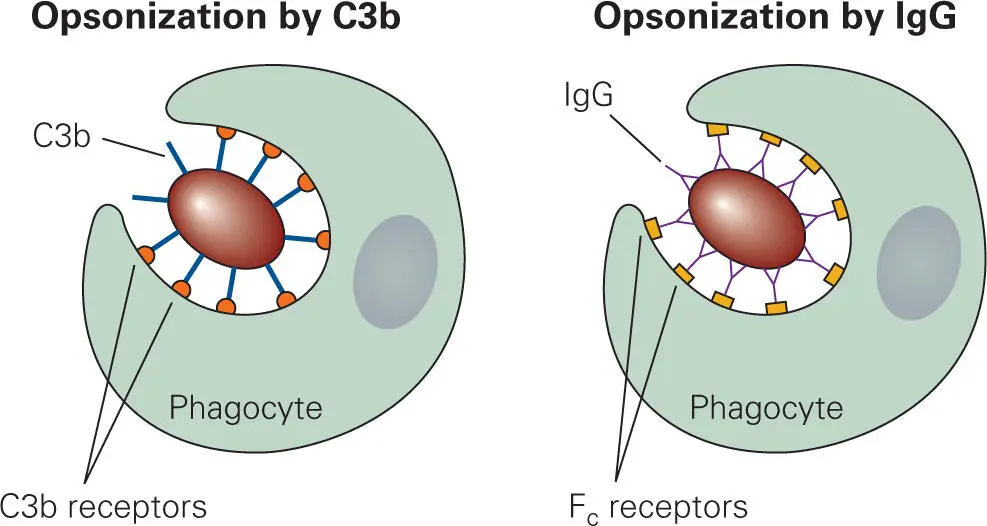
Figure 3-12. Opsonization of a bacterium by activated complement component C3b and antibodies. Combined opsonization by both C3b and antibodies considerably enhances the uptake of the bacterium by phagocytes. IgG, immunoglobulin G.
Activated complement components can also lead to direct killing of bacteria. Activated component C5b binds to the O-antigen of LPS of Gram-negative bacteria and recruits sequentially C6, C7, C8, and multiple C9 proteins to form a MAC in the bacterial membranes ( Figure 3-13). Formation of the MAC kills bacteria by creating holes in their membranes. Bacteria that can be lysed by MAC are said to be serum sensitive, i.e., the addition of serum-containing complement components to the bacteria will kill the bacteria. Some Gram-negative bacteria have altered LPS O-antigens that do not bind C5b and therefore cannot form a MAC on their surfaces, while others have extra-long LPS O-antigens that can still bind C5b but cannot form a MAC in the bacterial membrane. Gram-negative bacteria that cannot be lysed by MAC are said to be serum resistant, i.e., they are resistant to killing by serum complement via MAC formation. Gram-positive bacteria are inherently serum resistant, because their thick cell walls prevent MAC assembly at the cytoplasmic membrane.
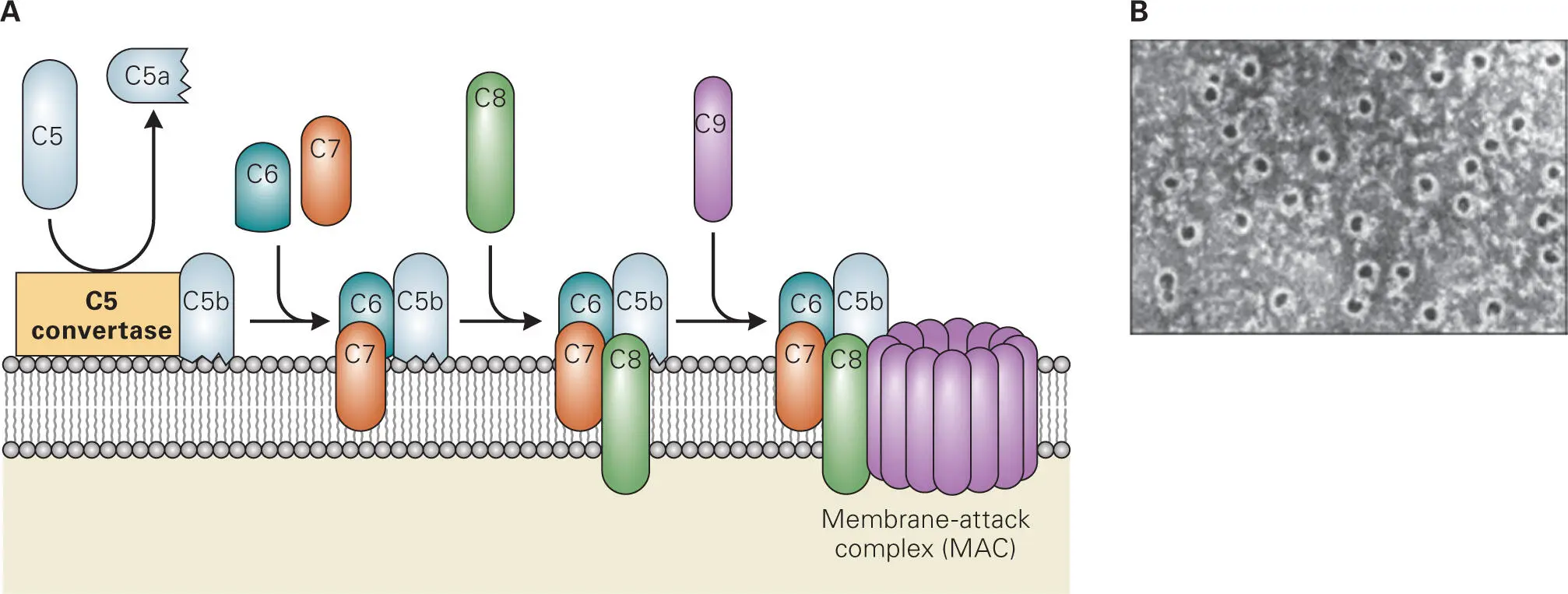
Figure 3-13. Formation of the membrane attack complex (MAC). (A) C5-convertase assembled on the bacterial cell surface cleaves C5 to generate C5a, which is a chemokine for phagocytes, and C5b, which binds to the O-antigen of LPS on Gram-negative bacteria and recruits C6 through C8 to initiate MAC formation and insertion into the membrane. Up to fifteen C9 proteins then oligomerize into the complex. (B) MAC pores in the membrane that then kills the bacteria. Reproduced from Janeway C, Travers P, Walport M, Shlomchik M. 2004. Immunobiology, 6/e. Garland Science, New York, NY, with permission.
Steps in Complement Activation
The steps in complement activation by each of the three pathways are shown in more detail in Figure 3-14. The classical complement pathway (called that because it was the first to be discovered) is initiated when the Fc regions of several antigen-bound IgG molecules or one pentameric IgM molecule bind to the surface of a bacterium and are subsequently cross-linked by C1, a multi-protein complex of hexameric C1q, two C1r and two C1s zymogens (C1qr2s2) ( Figure 3-14A). C1q binds directly to the heavy chain of antibodies bound to the bacterial surface and activates the two C1r serine proteases, which then in turn cleave and activate the two C1s serine proteases. Activated C1s cleaves C4 into C4a and C4b. C4b, like C3b, has a reactive thioester group that enables it to covalently attach to the bacterial surface at a site near the C1qr2s2 complex. C1s also cleaves C2 into C2a and C2b. C4b binds to C2a to form the C3 convertase (C2aC4b). C3 convertase then cleaves C3 to C3a, which diffuses away from the site, and C3b, which covalently binds to the bacterial surface.
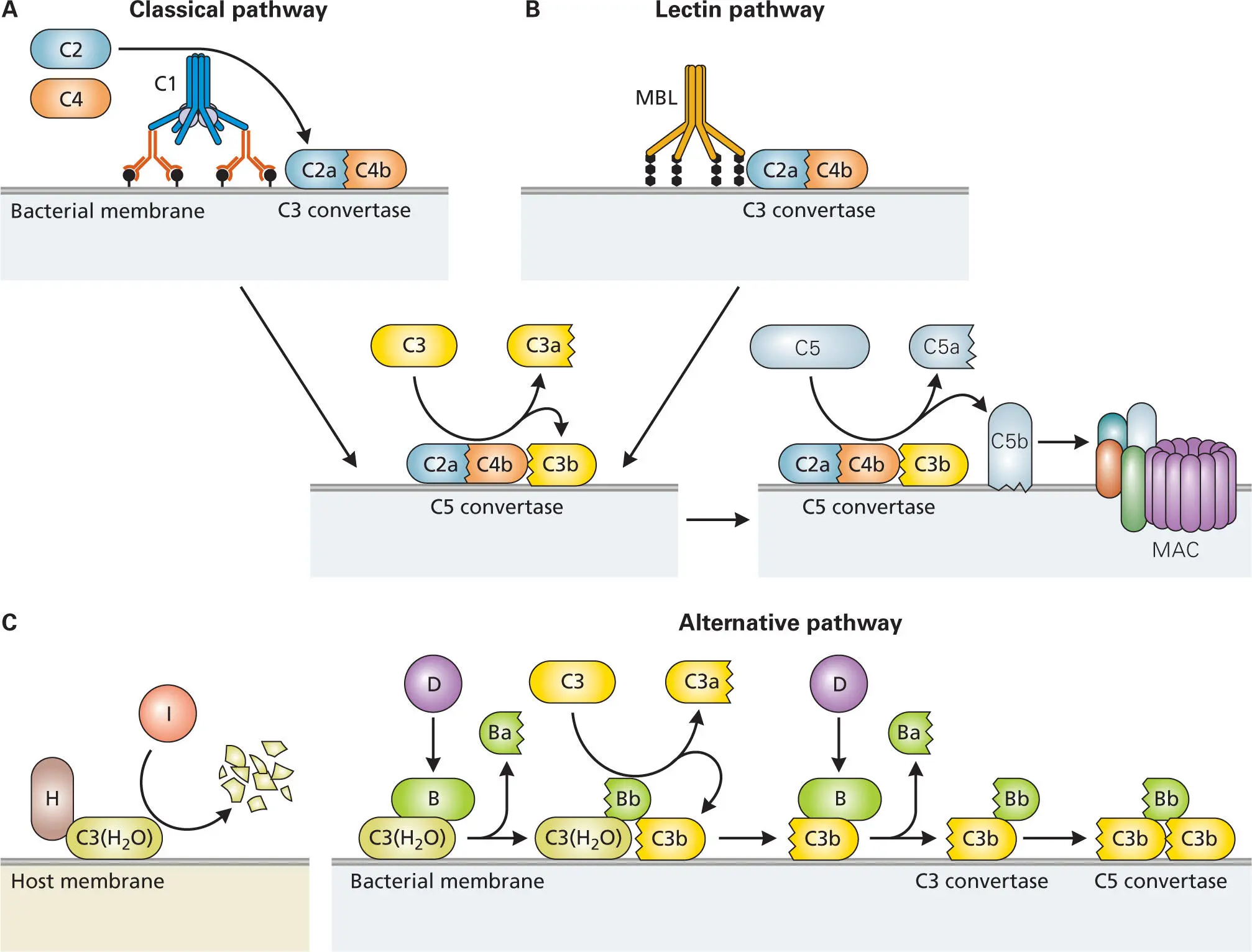
Figure 3-14. Activation of complement by the classical, lectin, and alternative pathways. (A) Classical pathway: Two IgG molecules or one IgM molecule attached to the surface of a bacterium bind complement component C1, causing an autoproteolytic event that activates it. C1, C4b, and C2a bind to each other and to the bacterium’s surface to form C3 convertase. The addition of C3b produces C5 convertase that triggers assembly of the MAC. (B) Lectin pathway: The mannose-binding lectins (MBLs) activate the complement pathway similarly to antibodies, except that they interact with C4 and C2 rather than C1. After that point, this pathway is the same as the classical pathway. (C) Alternative pathway: C3-H2O, an activated form of C3 that resembles C3b in conformation, is normally produced at low levels. If it binds a host cell surface through serum factor H, then the H-bound C3-H2O complex is targeted for destruction by serum protein I. If C3-H2O binds to the surface of a bacterium, it can form a complex with factor B, which is targeted for cleavage by factor D to generate Ba and Bb. The C3-H2O complexed with Bb then cleaves more C3 into C3a and C3b. The C3b then covalently binds to the bacterial surface, where it binds factor B, which gets cleaved by factor D to form C3bBb (C3 convertase) on the bacteria surface. Addition of more C3b produces C5 convertase. C5 convertase triggers assembly of the MAC.
Читать дальше















