Figure 3-5summarizes the different TLRs in humans and mice and where they are localized in the cell, as well as the signaling pathways that they activate. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are exposed on the surface of the phagocytic cell, whereas TLR3, TLR7, TLR8, and TLR9 reside within endosomes. Each TLR contains a leucine-rich repeat (LRR) domain that is extracellular for the surface TLRs or faces into the lumen of the vesicle for the endosomal TLRs. The LRR domain binds the PAMP ligand(s) and induces dimerization. The intracellular domain of each TLR has a conserved region of amino acid homology with interleukin-1 receptors (IL-1Rs), referred to as the Toll/IL-1R (TIR) domain. The TIR domain conveys the signal detected by the LRR domain binding to a PAMP ligand to the interior of the phagocytic cell through a signal transduction cascade mediated through adaptor proteins and kinases, including the myeloid differentiation primary response gene 88 protein (MyD88), IL-1R-associated kinases (IRAKs), transforming growth factor β-activated kinase 1 (TAK1), TAK1-binding protein 1 (TAB1), TAB2, tumor necrosis factor receptor-associated factor 6 (TRAF6), and others. In all cases, activation of these signaling pathways leads to expression of inflammatory genes and induction of phagocytic functions, such as maturation and secretion of cytokines and stimulation of the oxidative burst that kills opsonized bacteria.
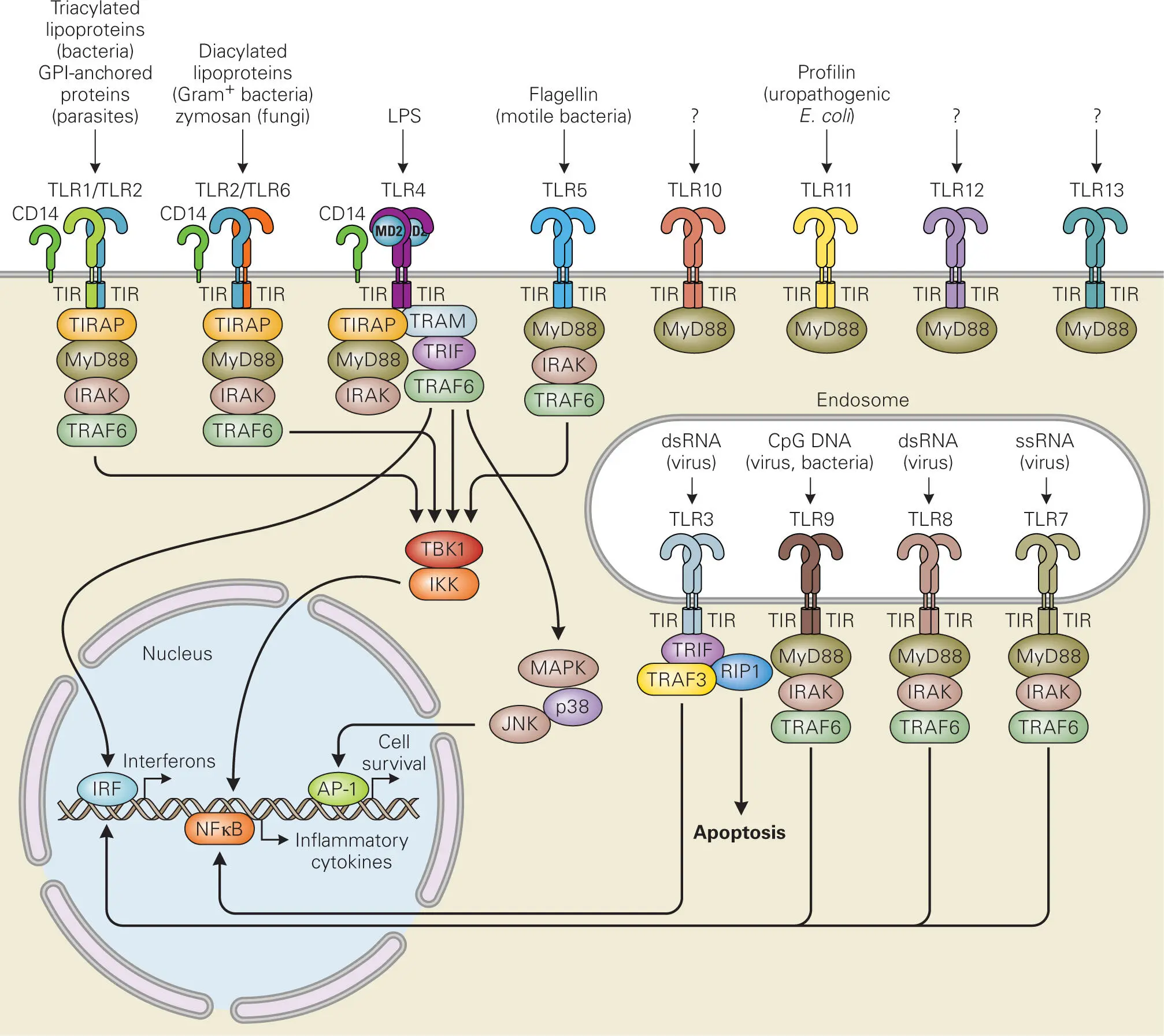
Figure 3-5. The Toll-like receptor (TLR) family and their signaling pathways in humans and mice. TLRs mediate the activation of the innate immune system through recognition of pathogen-associated molecular patterns (PAMPs). TLR1, TLR2, and TLR6 form heterodimers on the plasma membrane that bind to microbial membrane components. TLR4 forms homodimers that bind with MD2, CD14, and LPS bound LBP on the plasma membrane (see Figure 3-6). TLR5 forms homodimers on the plasma membrane that bind bacterial flagellin. TLR3, TLR7, TLR8, and TLR9 form homodimers inside endocytic vesicles that recognize various forms of nucleic acids. The ligands for TLR10, TRL12, and TLR13 are unknown. Humans have a mutated TLR11 gene that does not express protein, and do not have genes for TLR12 or TLR13. In mice TLR11 is functional and recognizes parasites and uropathogenic bacteria. TLRs activate downstream signaling pathways that lead to expression of proinflammatory cytokines through various adaptor proteins (MyD88, TIRAP, TRAM, TRIF, IRAK, TRAF6, TRAF3, and RIP-1) that interact with their intracellular TIR domains, which have homology with the receptors for IL-1 and IL-18.
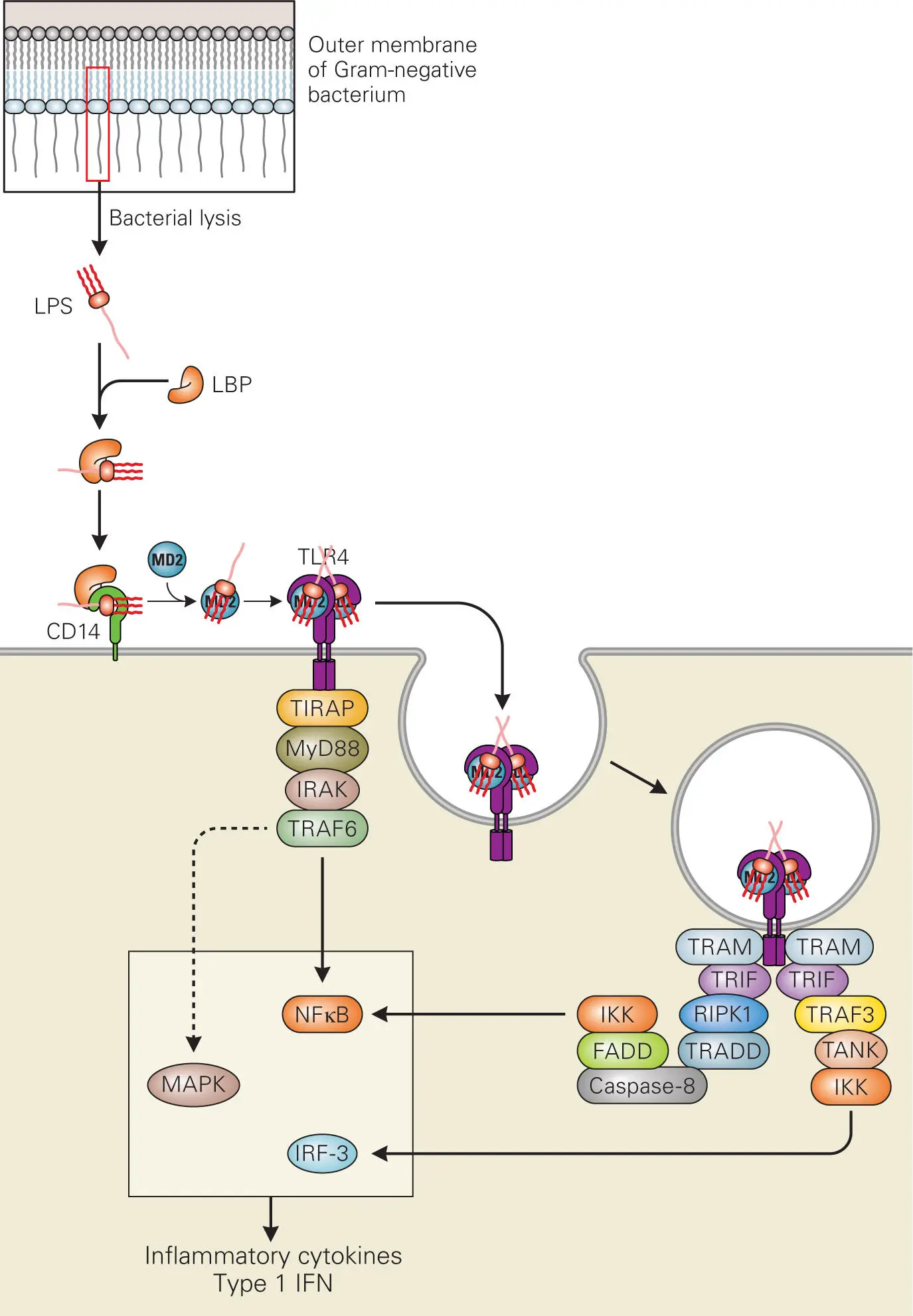
Figure 3-6. Cellular recognition of LPS through Toll-like receptor signaling.
The process by which bacterial components (PAMPs) trigger cytokine release through TLR signaling is best understood in the case of LPS (see Box 1-4). LPS that is released from outer membranes due to bacterial lysis binds to LPS-binding protein (LBP), a blood protein produced by the liver, and is delivered to CD14, a protein receptor on the surface of macrophages and other cytokine-producing cells ( Figure 3-6). LPS is then transferred to the transmembrane signaling receptor TLR4 and its accessory protein MD2. This TLR4/LBP/CD14/MD2 complex triggers a cellular signal transduction pathway that leads to activation of the transcription factor NF-κB, which then moves to the nucleus and induces inflammatory cytokine gene expression.
The host cell also has a PAMP detection system for intracellular pathogens, called NOD-like receptors (NLRs). Humans have twenty-two different NLR proteins that sense a variety of PAMPs, including cell wall components of intracellular bacteria, toxins, and host-derived ligands (such as damaged membrane components). NLRs consist of three domains: a variable N-terminal domain with different sensor-like functions (depending on the type of NLR); a central nucleotide-binding domain that mediates ATP-dependent auto-oligomerization (called a NACHT domain); and a C-terminal LRR domain that functions analogously to that of TLRs to detect bacterial-derived PAMPs inside host cells (peptidoglycan components in the case of NOD1 and NOD2, flagellin in the case of NAIP and NLRC4, and anthrax toxin in the case of NLRP1). Activation of most NLRs leads to NLR oligomerization and formation of the inflammasome, a complex of multiple signaling (NLR, caspase-1) and adaptor (ASC) proteins that mediate oligomerization and self-cleavage of pro-caspase-1, a zymogen (a proenzyme that is inactive until a proteolytic cleavage event converts it into its active form) ( Figure 3-7).
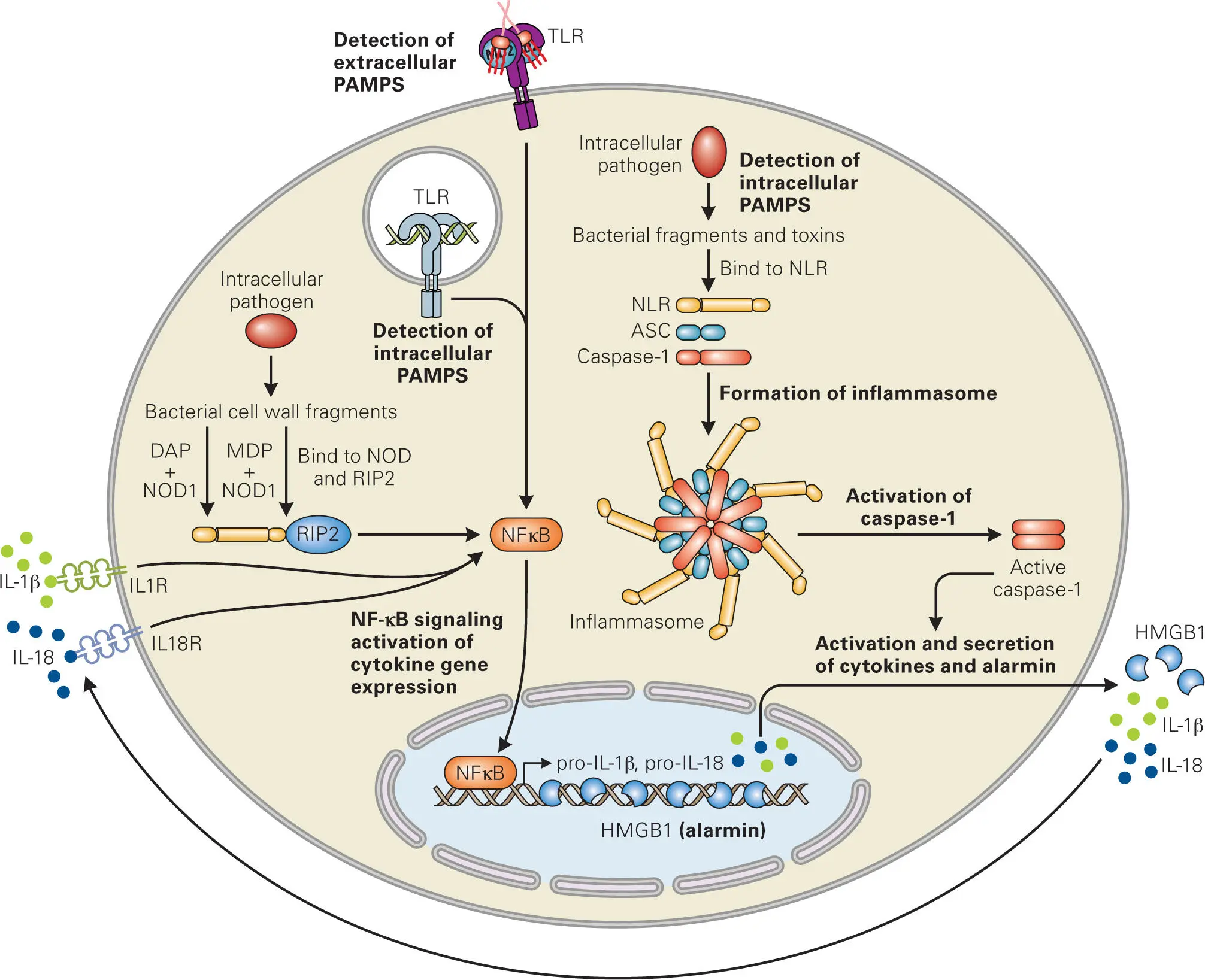
Figure 3-7. NOD-like receptor (NLR) sensing of intracellular pathogens. PAMP activation of TLRs and binding of the proinflammatory cytokines (IL-1β and IL-18) to their cognate receptors stimulate NF-κB signaling that leads to expression of pro-IL-1β and pro-IL-18. Infection with intracellular pathogens induces assembly of the NLRs, adaptor protein (ASC), and caspase-1 into inflammasome complexes, which lead to caspase-1 activation and subsequent cleavage and secretion of IL-1β and IL-18, as well as release of alarmin (HMGB1) and induction of pyroptosis. Unlike other NLRs, NOD1 binds the bacterial peptidoglycan derived D-Glu-meso-diaminopimelic acid (DAP), while NOD2 binds muramyl dipeptide (MDP) to form a nodosome complex, comprised of the ligand-bound NOD and a serine/threonine kinase adaptor protein, Rip2 (also called RIPK2), which in turn activates NF-κB. Adapted from Lamkanfi M, Dixit VM. 2011. J Immunol 187:597–602, with permission.
Caspase-1 signaling pathways lead to a form of programmed cell death, called pyroptosis, where cells produce cytokines, swell, and burst. Caspase-1 proteolytic activity leads to maturation of proinflammatory cytokines, especially interleukin-1β (IL-1β). Production of these cytokines leads to inflammatory responses in macrophages, such as Th1 responses and release of IFN-γ, tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6) and interleukin-8 (IL-8), and activation of NK cells.
The best-described members of the NLR family are the NOD1 and NOD2 proteins, which have an N-terminal caspase activation and recruitment domain (CARD). Human NOD1 detects a part of the peptidoglycan derived from degradation of the Gram-negative cell wall, whereas NOD2 detects a degradation product derived from Gram-positive peptidoglycan. While NOD1 is expressed by many cell types, NOD2 is expressed primarily in macrophages, DCs, Paneth cells, keratinocytes, and mucosal epithelial cells. Interestingly, NOD1 and NOD2 are unlike other NRLs in that they do not appear to form inflammasomes that directly activate caspase-1, but rather they form their own signaling complex, called a nodosome. The nodosome is directed to the site of bacterial entry into the cell, where it also interacts with the receptor-interacting serine/threonine-protein kinase 2 (Rip2/RIPK2) through the CARD domains. This is followed by recruitment of a number of other signaling molecules and potent activation of the transcription factor NF-κB, which is transported into the nucleus, where it induces expression of IL-1β, IL-18, and other proinflammatory genes.
In addition to sending out cytokine signals to alert the immune system of infection by pathogens, host cells that are severely damaged as a result of pathogen invasion also produce and release other endogenous host cell molecules, called alarmins. Alarmins are released into the extracellular medium by infected cells that are lysed or die by non-apoptotic mechanisms. One such alarmin is the high mobility group box 1 (HMGB1) protein, a nuclear protein that binds to nucleosomes and promotes DNA bending. Cells that are undergoing apoptosis modify their chromatin such that HMGB1 remains tightly bound to the DNA and not released, whereas cells that are dying by necrosis or lysis release HMGB1 into the extracellular medium. In response to PAMP detection and inflammatory stimulation, cells can also actively secrete HMGB1, which then serves as a chemokine to recruit phagocytes to the site of infection.
Читать дальше















