How Phagocytes Kill Bacteria
Oxidative Burst in Phagolysosomes
The steps involved in the killing of a bacterium by a phagocyte are shown in Figure 3-8. The phagocyte first forms pseudopods that engulf the bacterium. Phagocytosis (engulfment) requires dynamic rearrangements of actin, a major component of the eukaryotic cytoskeleton. After engulfment, the bacterium is encased in an endocytic vesicle called a phagosome. The phagosomal membrane contains ATPases that pump protons into the phagosome interior, reducing the internal acidity to as low as pH 4.5. Phagocytes also carry antibacterial proteins that are as toxic to the phagocytes and surrounding tissue cells as they are to their bacterial targets. Accordingly, these toxic proteins are stored in an inactive form in lysosomes. Fusion of a lysosome with a phagosome to form a phagolysosome releases the lysosomal proteins into the phagolysosome interior, where they are activated by the low pH of the phagolysosome interior.
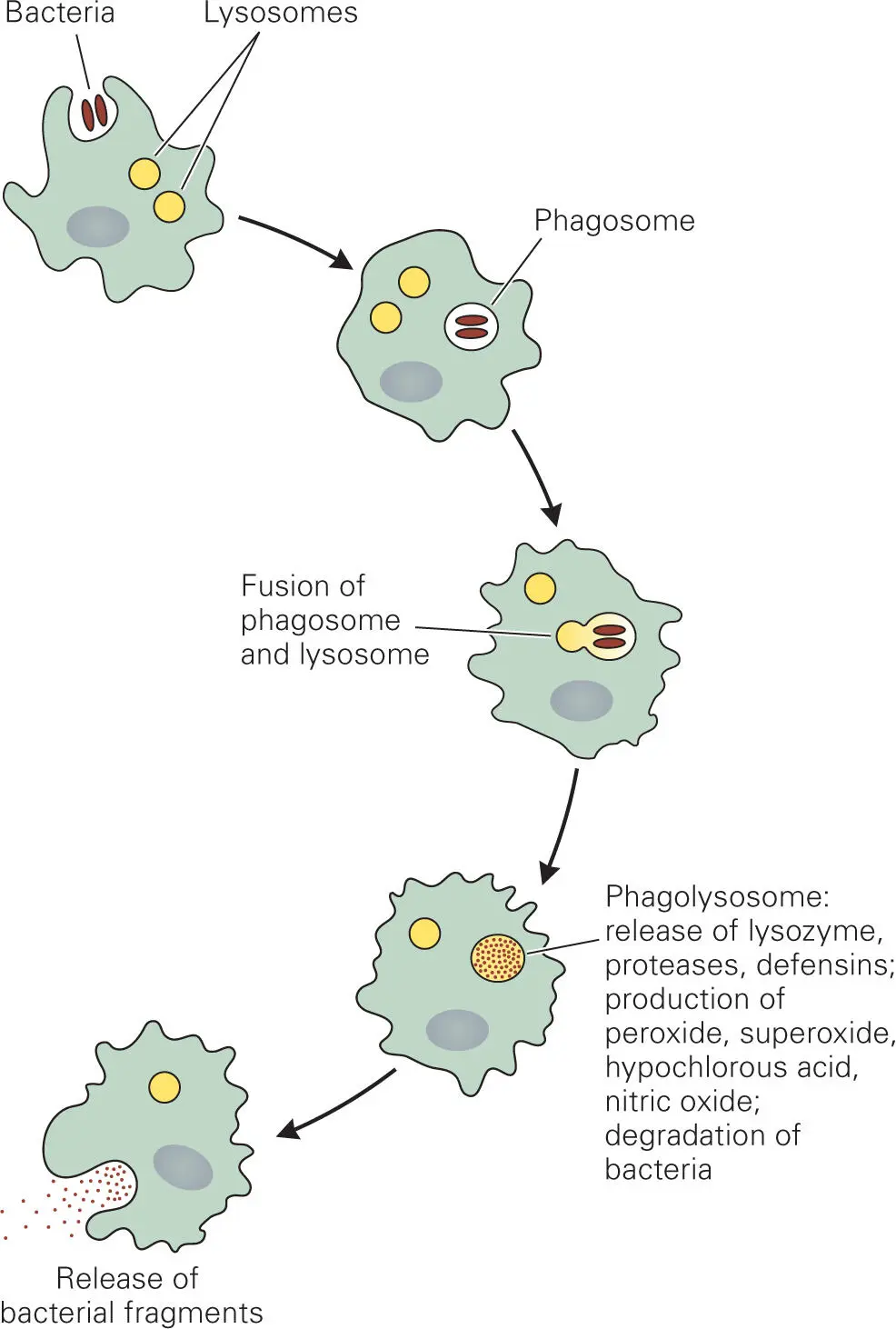
Figure 3-8. Steps in ingestion and killing of bacteria by phagocytes. Bacteria are first engulfed by endocytosis into a phagosome. Fusion of phagosomes and lysosomes releases toxic enzymes and proteins that kill most bacteria. Debris from dead bacteria is then released by exocytosis.
Phagocytes possess two general types of bacterial killing mechanisms mediated by the fusion of phagosomes and lysosomes: non-oxidative and oxidative. Lysosomal vesicles contain various macromolecule-degrading enzymes (proteases, lipases, glycosidases, hydrolases, nucleases, and lysozyme) that destroy bacterial components and mediate nonoxidative killing. Degrading enzymes, such as proteases, phospholipases, glycosidases, hydrolases, and lysozyme, destroy surface and membrane components of the bacteria. Nucleases hydrolyze bacterial DNA and RNA that are released during lysis. Other lysosomal proteins, such as defensins, antimicrobial peptides, and other membrane permeabilizers, insert into bacterial membranes and create pores that permeabilize the membrane, kill the bacteria, and allow the bacterial cytoplasmic components to leak into the surrounding environment.
Oxidative killing occurs through formation of toxic reactive oxygen and reactive nitrogen species. Lysosomes have another type of lysosomal protein, myeloperoxidase, that produces reactive forms of oxygen that are toxic to many bacteria (similar to lactoperoxidase). The generation of toxic forms of oxygen by phagocytes is called the oxidative (or respiratory) burst. Myeloperoxidase is only activated when it is brought into contact with an NADPH oxidase, which is located in the phagosomal membrane, and when the resulting complex is then exposed to the low pH of the phagolysosome interior. During an infection, cytokines stimulate increased production of these lysosomal enzymes, thus increasing the killing potential of the oxidative burst.
The reaction catalyzed by the myeloperoxidase complex has three steps ( Figure 3-9). First, NADPH oxidase generates a superoxide radical: NADPH + 2O2—> 2O2− (superoxide radical) + NADP+ + H+. The superoxide radical is extremely reactive and is readily converted into hydrogen peroxide (H2O2) by superoxide dismutase. Myeloperoxidase catalyzes the reaction of hydrogen peroxide with chloride (Cl−) or thiocyanate (SCN−) ions (as well as bromide or iodide ions) to form hypochlorite (−OCl, the active ingredient in bleach) or hypothiocyanite (−OSCN), respectively. These reactive oxygen molecules are toxic to bacteria because they inactivate essential bacterial surface proteins through oxidization of sulfur-containing amino acids and molecules, such as cysteine, methionine, and glutathione. For example, oxidation of the thiol groups (-SH) of the amino acid side chain of cysteine in proteins results in formation of sulfenic acid, sulfonic acid, or disulfide linkages.
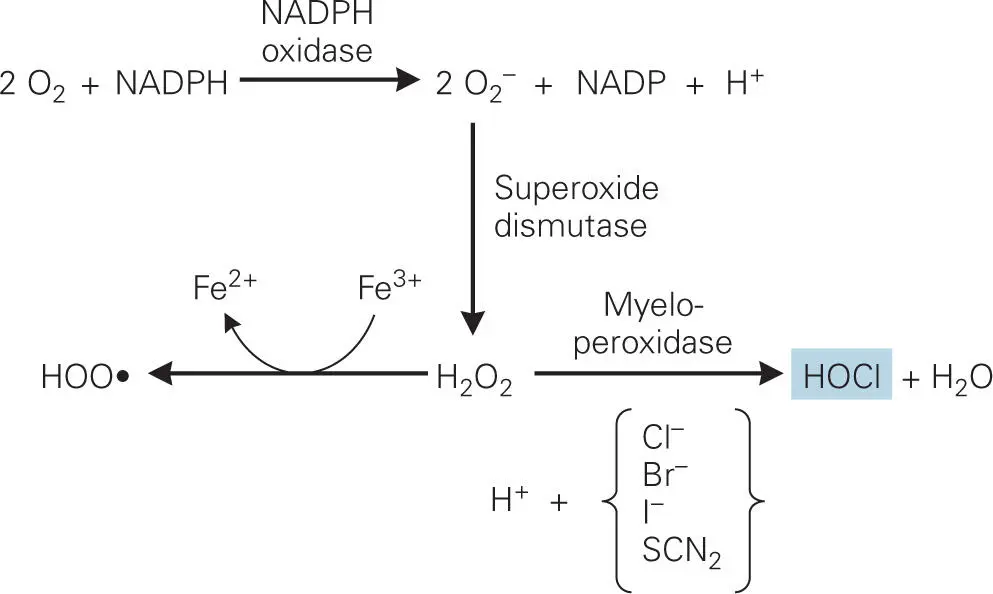
Figure 3-9. Oxidative burst in the phagolysosome. The phagosome contains two unique enzymes: lysosomal myeloperoxidase and phagosomal membrane-bound NADPH oxidase. Superoxide dismutase converts the superoxide radical (O2−) generated by NADPH oxidase into hydrogen peroxide (H2O2). Myeloperoxidase then catalyzes the reaction of hydrogen peroxide with chloride (Cl−), thiocyanate (SCN−), or other halide ions to form hypochlorite (−OCl), the active ingredient in bleach, or hypothiocyanite (OSCN−), both of which are extremely toxic to bacteria. Under these oxidative conditions iron ions exist predominantly in the oxidized ferric (Fe3+) state, and this free Fe3+ plus hydrogen peroxide forms hydroperoxyl radical (HOO•) via a nonenzymatic Fenton reaction, that can also damage macromolecules such as proteins and DNA.
Under these oxidative conditions iron ions exist predominantly in the oxidized ferric (Fe3+) state. Inside the body, Fe3+ ions are bound to iron-binding proteins, such as transferrin or lactoferrin, but in phagolysosomes the low pH environment causes the release of Fe3+ from the proteins. The freed Fe3+ ion plus hydrogen peroxide (H2O2) forms hydroperoxyl radical (HOO•) via the nonenzymatic Fenton reaction, which can also damage DNA.
Human monocytes and macrophages also produce another very simple but powerful antimicrobial compound, nitric oxide (NO). NO is toxic in its own right, attacking bacterial metalloenzymes, proteins, and DNA. Additionally, it can combine with superoxide to form peroxynitrite (OONO−), a very reactive molecule that oxidizes amino acids and is toxic to both bacteria and human cells. Synergistic reactions between NO and superoxide during the oxidative burst may help make the burst more toxic for bacteria. NO may also serve as a signaling molecule to regulate the functions of phagocytic cells, as well as other adaptive immune cells. NO has been implicated in so many areas of human and animal physiology that the journal Science chose it as the molecule of the year in 1992.
During an infection, cytokines induce NO synthesis by many human cell types. NO production is indicated by the presence of the stable end products of its oxidation, nitrite and nitrate, in the blood. NO may also contribute to some of the symptoms of disease, including vascular collapse and tissue injury, as mice lacking the inducible NO synthetase responsible for generating NO tolerate bacterial endotoxin with fewer toxic side effects than normal mice. On the other hand, these mice are highly susceptible to infections by intracellular bacterial and protozoan pathogens, such as Mycobacterium tuberculosis or Leishmania major, which cause tuberculosis or leishmaniasis, respectively. The human pathogen Neisseria meningitidis, which is responsible for meningococcal disease, has at least two NO detoxification enzymes that enhance survival during nasopharyngeal colonization and during phagocytosis by human macrophages.
Autophagy—Another Pathway for the Killing of Intracellular Pathogens
Some intracellular pathogens, such as Mycobacterium tuberculosis and Salmonella enterica serovar Typhimurium, are able to escape destruction through the phagolysosomal pathway by modifying the phagosome into a specialized vacuole that does not fuse with lysosomes. Others, such as Rickettsia conorii, escape the phagosome and reside within the host cytosol. In such cases of intracellular pathogenesis, another cellular pathway, called autophagy, is employed. Autophagy normally enables the breakdown and recycling of dysfunctional cellular components, such as mitochondria, by sequestering them from the rest of the cytosolic components into a double-membrane organelle called an autophagosome, which then fuses with a lysosome to destroy the contents of the resulting autophagolysosome.
Читать дальше














