The lectin pathway converges with the classical pathway in that it also stimulates cleavage of C4 and C2, with generation of C4b and C2a that form the C3 convertase complex ( Figure 3-14B). The collectin MBL circulates in the blood as a multimeric complex with one or more serine proteases, called MBL-associated serine protease (MASP) proteins and small MASP-associated protein (sMAP). MASPs can also bind to ficolins (FCNs). There are three known MASPs: MASP-1, MASP-2, and MASP-3. MASP-1 and MASP-2 resemble C1r and C1s, respectively. When MBL binds mannose groups of glycoproteins found on the surface of many pathogens, the MASP-2 protein cleaves C4 and C2 and initiates formation of the C3 convertase as in the classical pathway. MASP-1 can also cleave C3 directly, whereas MASP-3 appears to associate primarily with FCN but does not appear to activate complement, and its function remains unclear.
Activation via the alternative pathway (named because it was discovered after the “classical” pathway) bypasses the need for C1, C2, C4, antibodies, or MBL/MASP, and relies instead on C3b as the initiating component. As C3 circulates in blood and tissue, it is occasionally activated into a water-bound form (C3-H2O) that can interact with the serum proteins, factor B or factor H. Tissues of the body are coated with sialic acid residues, that can bind to one end of factor H. In the absence of a bacterial surface, C3-H2O binds to the other end of factor H to form a complex that produces C3b ( Figure 3-14C). This binding of C3b to factor H changes the conformation of C3b and targets it for proteolytic cleavage into iC3b by the serum protease factor I. In the absence of a bacterial surface, the iC3b bound to factor H is targeted for further destruction by factor I. However, iC3b can still covalently bind to a bacterial surface via its internal thioester group and as such can serve as an opsonin, even though iC3b can no longer form active C3 convertase.
If C3-H2O instead binds to serum protein factor B, then another serum protein factor D cleaves that B to Ba and Bb. The resulting complex of C3-H2O bound to Bb cleaves another C3 molecule to produce C3a and C3b, that then covalently binds to the bacterial surface. This membrane-bound C3b binds another factor B, and the complex C3b-B is then targeted for cleavage by factor D to form the C3 convertase C3bBb, which in turn continues to cleave more C3 to make more C3a and C3b. C3bBb, which normally has a relatively short half-life of about 90 minutes, is further stabilized by binding of the protein properdin (P), which increases the half-life of the resulting C3bBbP complex by up to ten-fold. Properdin is a soluble glycoprotein released by PMNs, monocytes, and adaptive immune cells in response to the presence of proinflammatory cytokines such as TNF-α.
Generation of large amounts of C3b has two functions. A portion of the C3b binds to the bacterial surface and acts as an opsonin to enhance uptake by phagocytes, while another portion binds to the existing C3 convertase complexes (C3bBb or C2aC4b) to form the C5 convertase complexes (C3bBbC3b or C4bC2aC3b), that then cleave C5 into C5a and C5b. C5a diffuses away from the site and acts as a chemokine to recruit phagocytes, whereas C5b binds to LPS on the surface of Gram-negative bacteria and recruits C6, C7, C8, and C9 to form the MAC. Some bacteria produce a polysaccharide surface coating, called a capsule, that preferentially binds serum factor H rather than factor B. As a result, C3b is eliminated as it deposits on the surface, effectively preventing opsonization of the bacterial surface, as well as MAC formation.
Controlling Complement Activation
In all three pathways, it is important to keep the accelerated production of C3a, C3b, C5a, and C5b under control so that overstimulation of the inflammatory response does not occur and host cells are not damaged. To this end, several mechanisms protect host cells from complement over-activation ( Figure 3-15). Excess C3b molecules that bind to factor H when bound to sialic acid groups on the surface of host cells are proteolytically cleaved to produce iC3b. As mentioned previously, while iC3b is an effective opsonin ( Figure 3-15A), it can no longer aid in the formation of a C3 convertase or C5 convertase. Likewise, when iC3b is bound to factor H on host cell surfaces, it is subject to further degradation by serum factor I.
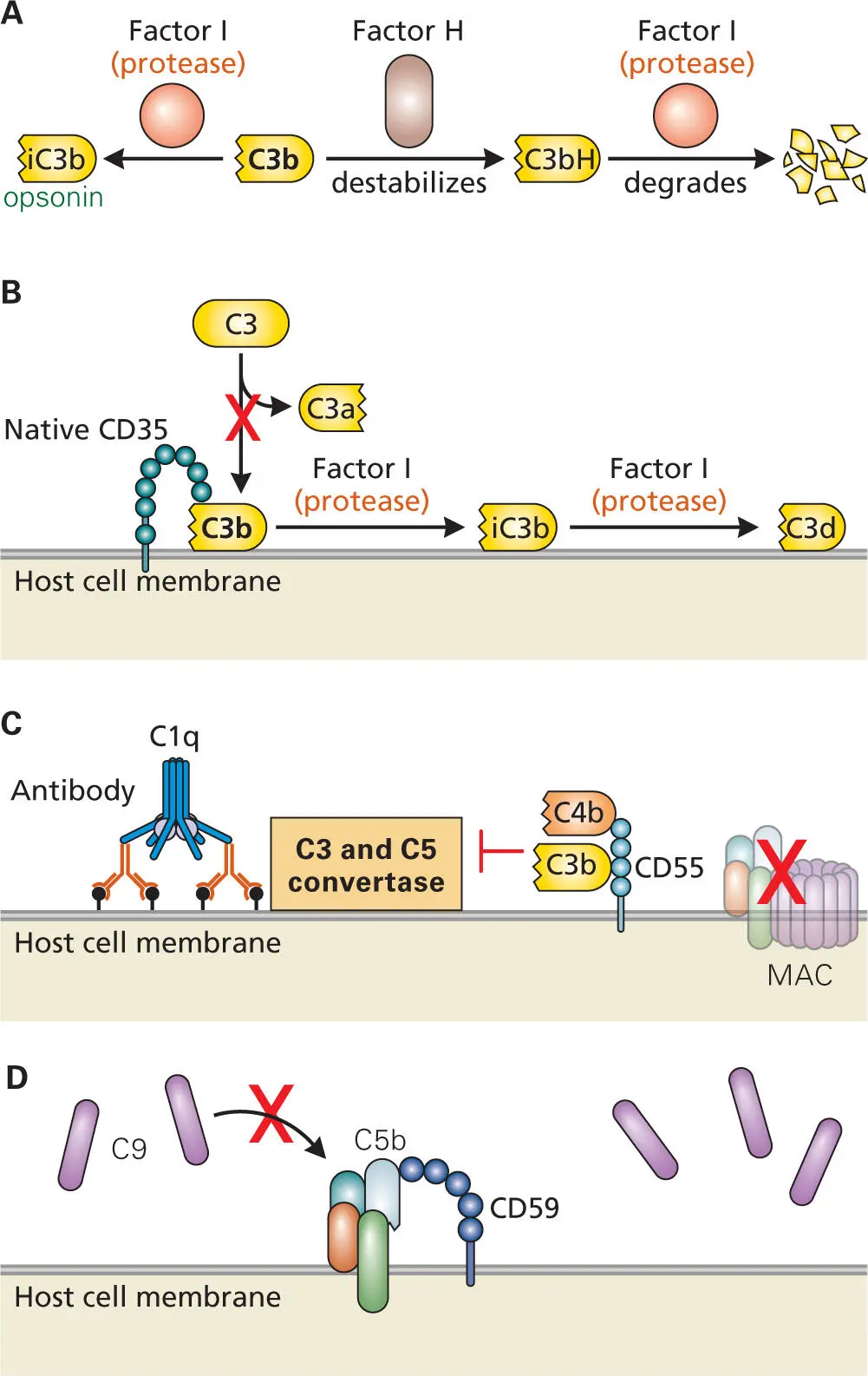
Figure 3-15. Controlling complement. Multiple regulatory pathways help to dampen complement activation, returning the signaling pathways to the resting state. (A) Factor H and factor I work together to limit the amount of C3b present in the circulation. Factor H competes with factor B for binding to C3b. Formation of complex C3bH destabilizes C3b, exposing a site for the protease activity of factor I to then completely degrade C3b. C3b can also be clipped to form iC3b, which still acts as an opsonin but no longer stimulates complement. iC3b also is degraded by factor I. (B) CD35 (CR1) binds to complement component C3b (as well as C4b) and dissociates the C3 convertase complex, thereby preventing further cleavage of C3 to C3a and C3b. CD35 is also a cofactor for factor I, which clips C3b (as well as C4b) to generate iC3b (and iC4b) and further degradation products. (C) CD55 binds to C3b and C4b and causes dissociation of the C3 and C5 convertase complexes, thereby preventing further cleavage of C3 and C5 and limiting the formation of C3a, C3b, C5a, and C5b–C9 (MAC). (D) CD59 binds the C5b–C8 complex, which is inserted into the cell membrane, and thereby blocks the binding of C9 to the complex and the polymerization of C9 to form membrane pores.
Once the pathogen has been cleared from the system and the heightened immune responses are no longer needed, the host cell uses additional regulatory proteins to help inhibit the complement pathways and return them to basal levels of activity. Several proteins protect host cells from complement-mediated damage by rapidly inactivating components of the complement pathways ( Figure 3-11). CD35 (complement receptor 1, CR1) is a membrane glycoprotein found on phagocytic cells that binds to opsonins on bacterial cells and mediates their phagocytosis ( Figure 3-15B). CD35 thus serves as the primary pathway for processing and clearing complement-opsonized immune complexes and inhibits both classical and alternative pathways. C4b-binding protein (C4BP), a glycoprotein found in blood plasma that binds C4b (and, to a lesser extent, C3b), is an inhibitor of the classical and lectin complement pathways. C4BP serves as a cofactor of serum factor I, accelerating the degradation of C3 convertase by factor I-mediated cleavage of C4b and C3b. CD46 (membrane cofactor protein) is also a membrane protein receptor that binds factor I as a cofactor to cleave and inactivate C3b and C4b.
Several factors help protect the host cell from being damaged by complement activation by blocking MAC from forming on the host cell surface and thereby protecting the host cell membrane ( Figure 3-15Cand D). CD59 (MAC-inhibitory protein, MAC-IP) is a membrane-associated glycoprotein that blocks C9 polymerization and MAC formation. CD55 (complement decay-accelerating factor, DAF) is a membrane-associated glycoprotein found on the surface of many blood cells. CD55 binds to C4b and C3b and interferes with their ability to bind C2b and Bb to prevent formation of the C3 convertases (C4bC2b and C3bBb, respectively) on the host cell surface, thereby protecting the host cell membrane.
Cytokines and Chemokines—Mediators of Immune Responses
Roles of Cytokines and Chemokines in Directing Innate Immune Responses
Cytokines and chemokines play a central role in regulating the cellular activities of both the innate and adaptive defense systems ( Table 3-2). These signaling molecules act as messengers by binding to receptors on the cells whose activities they direct. Cytokines are soluble glycoproteins (see Box 3-1) of 8 to 30 kDa produced by a variety of cells, including PMNs, DCs, NK cells, endothelial cells, and cells of the adaptive immune system, as well as other host cells when they are infected. Chemokines are small glycopeptides of 8 to 10 kDa that are produced by the same cells that produce cytokines. Their main function is to attract and activate phagocytes, a function similar to that of complement components C5a and C3a.
Читать дальше













