Box 3-1.
Glycoproteins
The surfaces of human cells are coated with glycoproteins that, as the name implies, contain proteins covalently linked to oligosaccharides. These glycoproteins play diverse roles in eukaryotic organisms. Shown are the structures of a typical N-glycosylation structure on human glycoproteins, that are capped with the nine-carbon monosaccharide sialic acid, called neuraminic acid. The predominant sialic acid found in mammalian cells is N-acetylneuraminic acid (core structure shown below). Not surprisingly, bacteria produce enzymes on their surfaces that can hydrolyze and release the sugars from host glycoproteins, such as the proteins NanA, BgaA, and StrH from S. pneumoniae, and provide food to the bacteria as well as opening up binding sites for bacteria to attach to the host cell surface.
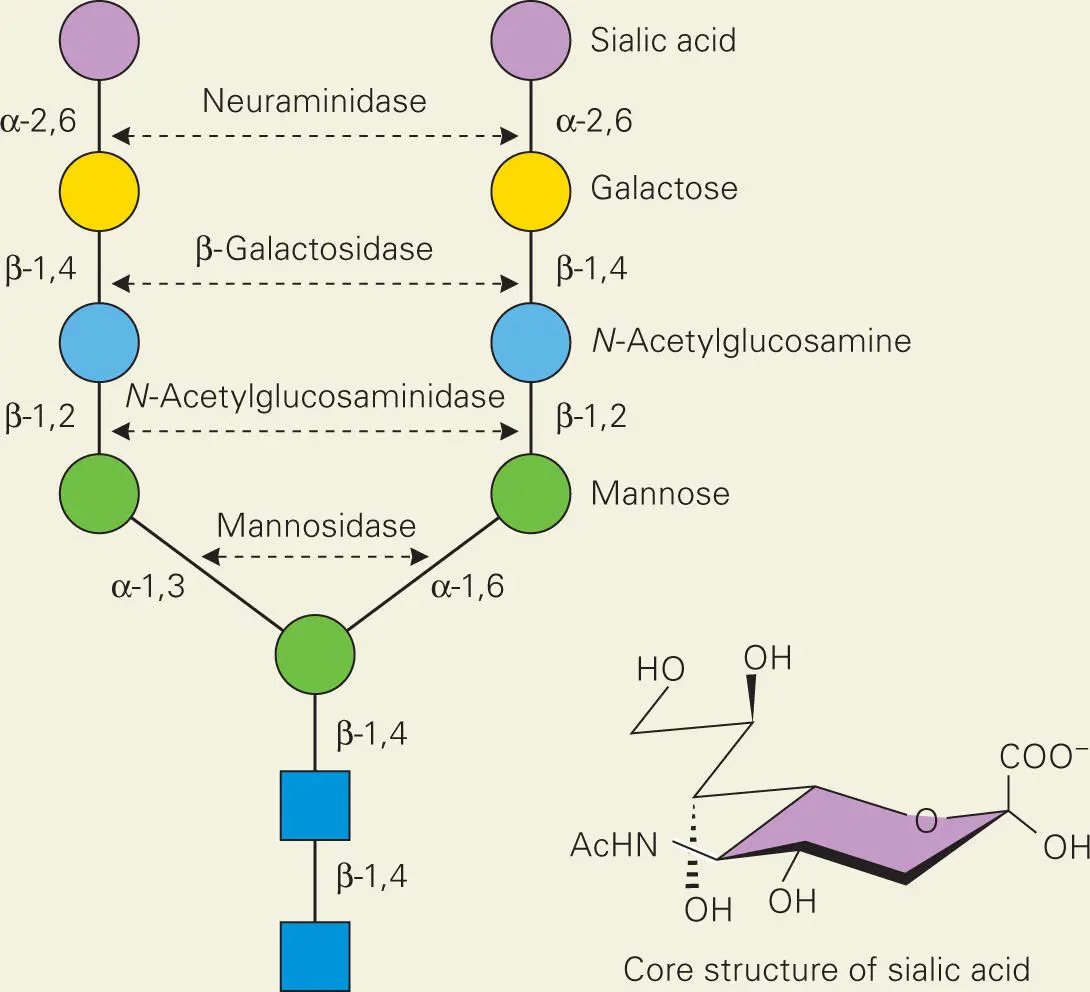
Source:
King SJ, Hippe KR, Weiser JN. 2006. Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol Microbiol 59:961–974 [PubMed] [CrossRef].
Cytokines and chemokines recognize and bind to specific receptors on the surfaces of target immune cells, that set off signal transduction cascades that modify the functions of the immune cells. Just as complement can be activated by bacterial surfaces, cytokine release can be triggered by interaction between cytokine-producing cells and molecules on the surfaces of the invading bacterium. In the case of Gram-negative bacteria, the outer membrane LPS that activates complement is also a molecule that stimulates cytokine production. Although the surface molecules of other types of bacteria that activate complement and stimulate cytokine release have not been studied nearly as well, it appears likely that the same surface molecules on these bacteria that activate complement also stimulate cytokine production.
During an infection, phagocytes and other cells (such as endothelial cells) release a variety of different cytokines. Some appear early in the infection and are responsible for up-regulation of innate defenses. Others appear late in the infection and help to down-regulate the defense response. Among the earliest-appearing cytokines are granulocyte-macrophage colony stimulating factor (GM-CSF) and interleukin-3 (IL-3). These cytokines stimulate stem cells to produce monocytes and granulocytes (especially PMNs) and trigger their release from the bone marrow into circulation. Other early-appearing cytokines, such as TNF-α, IL-1, IFN-γ, and interleukin-8 (IL-8), stimulate the monocytes and granulocytes to leave the bloodstream and migrate to the site of infection. The steps in this process for PMNs are illustrated in Figure 3-2.
Normally, PMNs move rapidly through the blood vessels, occasionally colliding with one of the vessel walls. TNF-α, IL-1, and IFN-γ stimulate endothelial cells to produce a set of surface proteins called selectins. These selectins bind to proteins on the surface of PMNs and other phagocytic cells in the blood, causing them to loosely attach to the blood vessel endothelium. This loose attachment slows the movement of the blood cells, causing them to roll along the endothelial surface. As this occurs, other selectins will appear on the endothelial cells, while IL-8 stimulates the PMNs to produce proteins called integrins on their surfaces. The integrins bind another set of cytokine-stimulated proteins on the endothelial cell surface, the intercellular adhesion molecules (ICAMs), to generate a tighter attachment between PMNs and endothelial cells. This tight association stops the movement of the PMNs and causes them to flatten against the blood vessel wall. The slowing and stopping of the PMNs is called margination. The PMNs then force themselves between endothelial cells, a process that is assisted by a PMN protein called platelet-endothelial cell adhesion molecule (PECAM). The proinflammatory complement components C3a and C5a assist in the process of transmigration (diapedesis, or extravasation) from the bloodstream into tissue by causing mast cells to release vasoactive histamine and heparin, which dilate blood vessels and make them leakier. Dilation of blood vessels is also assisted by the cytokine platelet-activating factor (PAF). In addition to histamine and heparin, PAF triggers mast cells to produce a number of other vasoactive compounds from the membrane lipid, arachidonic acid, including various leukotrienes and prostaglandins. Once the PMNs have moved out of the blood vessel and into surrounding tissue, a gradient of soluble complement components, chemokines or bacterial peptides, leads them to the site of infection (through chemotaxis).
As PMNs move through tissue, the proinflammatory cytokines TNF-α, IL-1, IL-8, and PAF also activate the oxidative burst response in PMNs so that the PMNs arrive at the infection site with their full killing capacity in place. Monocytes and the macrophages into which they develop similarly activate as they move into an infected area. IFN-γ further stimulates the killing ability of macrophages, producing activated macrophages. Because the signals that control the activities of PMNs, cytokines, and activated complement components are at their highest concentrations near an infected area, PMNs will exit the blood vessel near the location of infection rather than in other areas of the body. Additionally, the fact that activation of the phagocytes occurs only as they are moving into the infected area minimizes the amount of collateral damage to tissues outside the infected area.
The end result of the signaling pathways just described is that a high number of PMNs and other phagocytic cells will leave the blood vessels near the site of infection. However, there are some underlying conditions that will reduce the effectiveness of this signaling system and reduce PMN transmigration, including steroid use, stress, hypoxia, and alcohol abuse. Their inhibitory effect on transmigration may explain why these underlying health conditions are frequently associated with increased susceptibility to infection.
If the phagocytes are successful in eliminating the invading bacterium, other cytokines begin to predominate. In addition to the inhibitory regulators of the complement cascade mentioned earlier, a number of anti-inflammatory cytokines, such as IL-4, IL-10, and interleukin-13 (IL-13), down-regulate production of TNF-α and reduce the killing activities of phagocytes, thereby countering the proinflammatory response and allowing the phagocyte defense system to return to its normal, relatively inactive level.
Inflammation and Collateral Damage
Inflammation, derived from the Latin word inflammare, which means “to set on fire,” is the immunologic and vascular response of the body to invasion by pathogens. Inflammation is caused by the release of proinflammatory cytokines, including C3a and C5a generated by the complement cascade; tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) released by basophils, mast cells, activated DCs, macrophages, and other phagocytes; and subsequent production of prostaglandins and leukotrienes. Other inflammatory mediators include the vasoactive peptides kallidin and bradykinin, which dilate blood vessels leading to a drop in blood pressure and increase vascular permeability at the site of infection to allow neutrophil and monocyte transmigration (extravasation).
Although inflammation serves to protect and control infections, it can also cause further tissue damage, which is manifested as the disease symptoms of redness, swelling, heat, and pain. The increased blood flow to the area due to vasodilation results in redness and elevated temperature. The increased vascular permeability causes blood fluids to leak out of the vessels as the phagocytes transmigrate, causing edema (swelling) of the surrounding tissue. The source of the pain is still not clearly understood, but it is likely caused by the combined effects of cytokines, prostaglandins, and coagulation cascade components on nerve endings in the inflamed region. Bradykinin also appears to increase sensitivity to pain. To counter the proinflammatory response, there are a number of anti-inflammatory cytokines, including IL-1 receptor antagonist, interleukin-4 (IL-4), and interleukin-10 (IL-10), that serve to regulate the immune response by inhibiting the action of the proinflammatory cytokines.
Читать дальше













