Thermal efficiency of this heat engine is
(1.89) 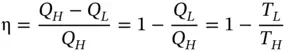
Since Eq. (1.89) is obtained without reference to a specific working fluid, it can be surmised that all reversible cycles operated between the same temperatures will have the same thermal efficiency.
Based on accumulated experimental knowledge, scientists and engineers have come to the conclusion that it is impractical to build the Carnot engine, and it remains to date as the ideal cycle against which real heat engine cycles are measured. If such an engine were to operate between combustion temperature of iso‐octane (gasoline) T H= 2300 K and the standard ambient temperature T L= 298.15 K , the Carnot efficiency would be 78%. By comparison, the most efficient reciprocating internal combustion engines can hardly achieve 50%.
1.3.8 Zeroth Law of Thermodynamics
If two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other and the three systems are said to be at the same temperature.
This law was added to the laws of thermodynamics early in the twentieth century because it was realised that the concept of equal‐in‐temperature is a prerequisite to a logical development of those laws. And to be logical, it was named the zeroth law of thermodynamics .
1.3.8.1 Thermodynamic Scale of Temperature
Temperature is a fundamental concept, not expressible in terms of other units or physical properties of the devices used to measure temperature, such as alcohol or mercury in glass thermometers or the electromotive force generated in a thermocouple. Physicists have established that temperature measures the kinetic energy of molecules, and the higher the molecular agitation, the higher the temperature and vice versa. The physicist William Thomson (Lord Kelvin) is credited with the establishment in 1848 of the absolute temperature scale (hence the symbol K for temperature) on the basis of Carnot's reversible cycle.
To show how it is possible to arrive at an absolute scale, a hypothetical experiment can be conducted to show that an absolute zero of temperature must exist, and then extend this line of reasoning to develop an ‘energy’ or ‘thermodynamic’ temperature scale.
It was shown earlier that the efficiency of the Carnot cycle is written as
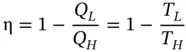
This equation shows that T Lcan never be zero or less than zero, for if T Lwere equal to zero, the thermal efficiency of the heat engine would be 1, or 100%, which would be a violation of the second law. Also, if T Lwere less than zero, the thermal efficiency would be greater than 100%, which would mean a reversal of the direction of Q L, and heat would be drawn from the low‐temperature source. This also would be a violation of the second law. So, theoretically, the absolute zero of temperature could never be reached.
If a Carnot engine is operated between a high‐temperature source at the temperature of boiling water T Hand a low‐temperature sink at the temperature of melting ice T L, and the amount of heat received Q steamand rejected Q icewere measurable accurately, the ratio Q steam/ Q icewould be equal to 1.3662. Since Q steam/ Q ice= T steam/ T ice,
(1.90) 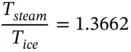
If the temperature range T steam− T iceis arbitrarily divided into 100 equal divisions (call it 100 ° C ), a second equation can be obtained
(1.91) 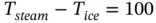
The simultaneous solution of Eqs. (1.90) and (1.91) yields the temperature of boiling water and melting ice on a thermodynamic scale:
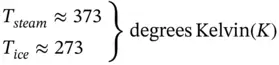
If the temperature range T steam− T iceis divided into 180 equal divisions (call it degrees Fahrenheit, F), we get
(1.92) 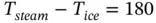

The determination of a thermodynamic scale using reversible Carnot engine as described here is practically impossible. To determine where the number 1.3662 comes from, we start with the equation of state for a perfect gas at constant volume and constant pressure, which yields
(1.93) 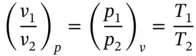
Next, constant‐volume and constant‐pressure thermometers are placed alternately in boiling water and melting ice, and the volume and pressure ratios of the gases in the devices (carbon dioxide CO 2, hydrogen H 2, and helium He ) are measured at an initial pressure. If the procedure is repeated for different initial pressures and the results plotted versus pressure, straight lines are obtained, which converge at zero absolute pressure to a value of 1.3662, which must be the ratio of absolute temperature of boiling water and melting ice.
Currently, the international temperature scale (ITS‐90) is the standard used to represent the thermodynamic (absolute) temperature scale as closely as possible with improved accuracy over gas thermometry.
1.3.9 Third Law of Thermodynamics
This law, based on empirical evidence, postulates that absolute entropy of a pure crystalline substance in complete internal equilibrium is zero at temperature zero degree absolute. The third law allows the determination of absolute entropies from thermal data.
The entropy change of a gas on molar basis is
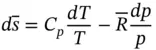
The entropy change for process 1–2 is then
(1.94) 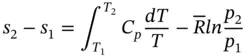
If the specific heat C pis assumed constant, Eq. (1.94) becomes
(1.95) 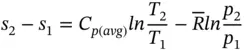
More accurate results can be obtained if the variability of specific heat with temperature is accounted for. Taking the absolute zero as the reference temperature, 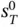 can be defined as
can be defined as
Читать дальше

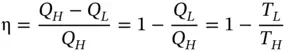
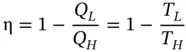
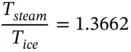
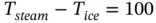
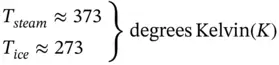
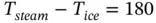

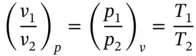
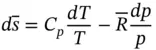
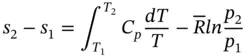
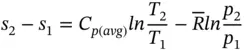
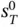 can be defined as
can be defined as








