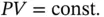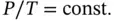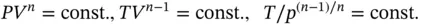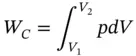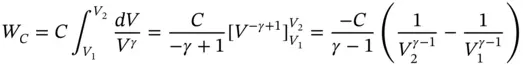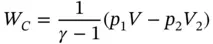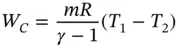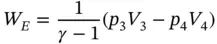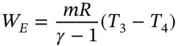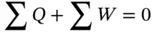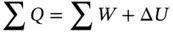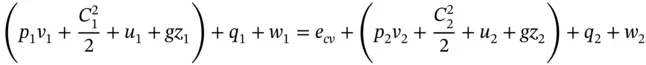1.3.3.3 Isothermal Process
An isothermal process takes place at constant temperature, and the equation of state can be written as
(1.51) 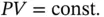
1.3.3.4 Isochoric Process
An isochoric process is a constant‐volume process for which the equation of state is reduced to
(1.52) 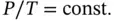
1.3.3.5 Polytropic Process
A process is referred to as polytropic when it deviates from the adiabatic as a result of heat crossing the boundary in addition to work. The relationship is similar to the adiabatic with the adiabatic exponent γ replaced by a polytropic exponent n ( n < γ ):
(1.53) 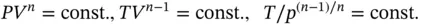
The volume V can also be written in terms of specific volumes v = V / m , which yields the additional equation p 1/ p 2= ( v 2/ v 1) n.
When a fluid undergoes a series of processes and then returns to its initial state, the fluid executes a thermodynamic cycle . A cycle that consists only of reversible processes is a reversible cycle.
To illustrate the application of some of the processes discussed earlier when calculating cycles, let us consider the theoretical thermodynamic cycle shown in Figure 1.8, which is known as the Diesel cycle in honour of the German inventor Rudolf Diesel.
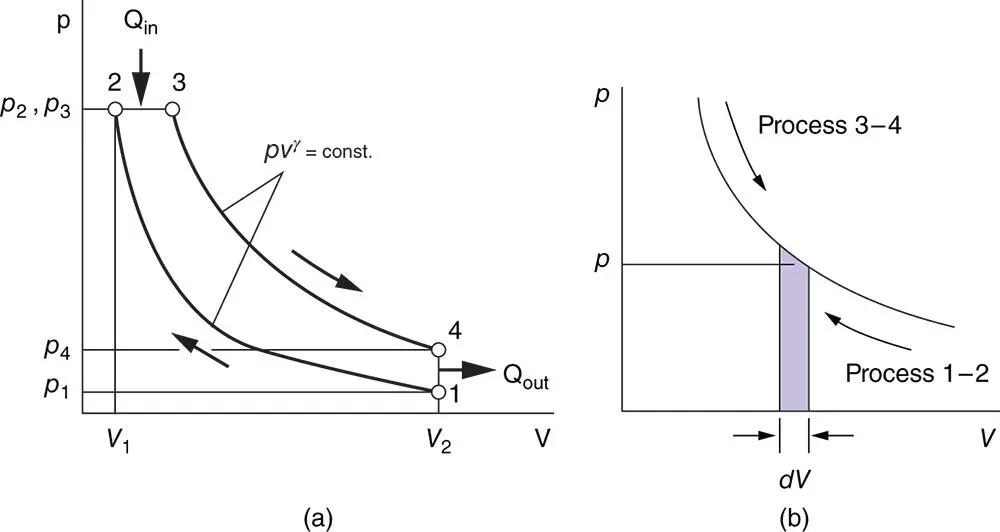
Figure 1.8Application of process equations in theoretical cycles: (a) Diesel cycle; (b) calculation scheme for compression and expansion work.
The cycle has two heat‐only processes and two polytropic processes:
Process 2 − 3: Constant‐pressure heat addition Qin = mcp(T3 − T2).
Process 4 − 1: Constant‐volume heat rejection Qout = mcv(T4 − T1).
Process 1 − 2: Adiabatic compression PVγ = C (C is the constant in Eq. 1.48).
The magnitude of the compression work done on the gas can be determined as shown in Figure 1.8b:
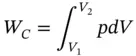
where
p : average pressure for the shaded area
dV : volume increment
Since p = C / V γ,
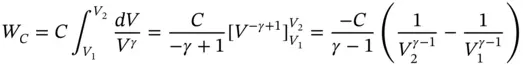
Now
C = p 1 V 1 γ= p 2 V 2 γ,
and hence,
(1.54) 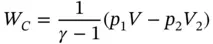
Since pV = mRT , the compression work can be rewritten as
(1.55) 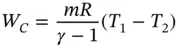
Process 3 − 4: Adiabatic expansion PV γ= C . The magnitude of the expansion work done by the gas can be deduced in a similar way:
(1.56) 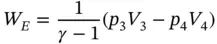
or
(1.57) 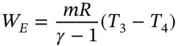
If the sign convention given in Figure 1.9is used, the compression work is negative work and the expansion work is positive work.
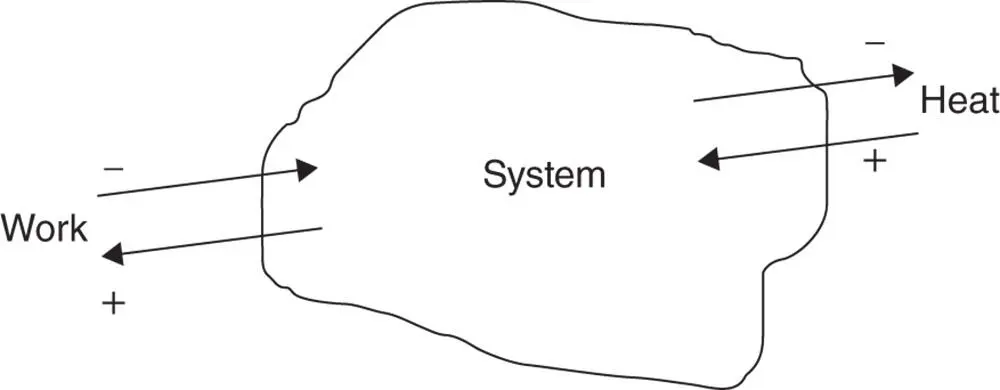
Figure 1.9Sign convention for heat and work.
1.3.5 First Law of Thermodynamics
This law is a statement of the law of conservation of energy: when a system undergoes a thermodynamic cycle, the net heat exchange ∑ Q between the system and its surroundings plus the net work exchange ∑ W between the system and the surroundings is equal to zero. This implies that energy can neither be created nor destroyed in a system; it can only be converted from one form to another:
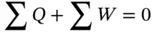
Considered here are applications of the first law in two engineering energy systems: non‐flow system and steady‐flow system.
1.3.5.1 Non‐Flow Energy Equation
For a closed system (no flow of fluid) that does not execute a cycle, the energy equation is
(1.58) 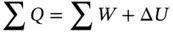
where
∑ Q : total heat transfer
∑ W : total work transfer
ΔU : internal energy change
The sign convention for work and heat is shown in Figure 1.9
1.3.5.2 Steady‐Flow Energy Equation
Consider 1 kg of a working fluid element that may be a liquid, gas, or vapour, or any combination, and which is flowing steadily through a control volume ( Figure 1.10). The fluid may possess energy in a number of forms between which conversions are possible:
1 Flow work or pressure work, given by pv.
2 Kinetic energy C2/2, due to the movement of the fluid element with velocity C.
3 Internal (thermal) energy u, due to the energy of the fluid molecules.
4 Potential or gravimetric energy, due to the height z above some datum line and given by zg.
5 Chemical, electrical, or magnetic energies may also be added, but these are not involved in the overwhelming cases encountered in thermal power cycles.
6 Heat Q may enter or leave the control volume.
7 Mechanical energy W may be added or removed, with some of the added energy being used to pump the fluid into the control volume or expel it out again.
8 Accumulated (stored) energy in the control volume as a whole ecv.
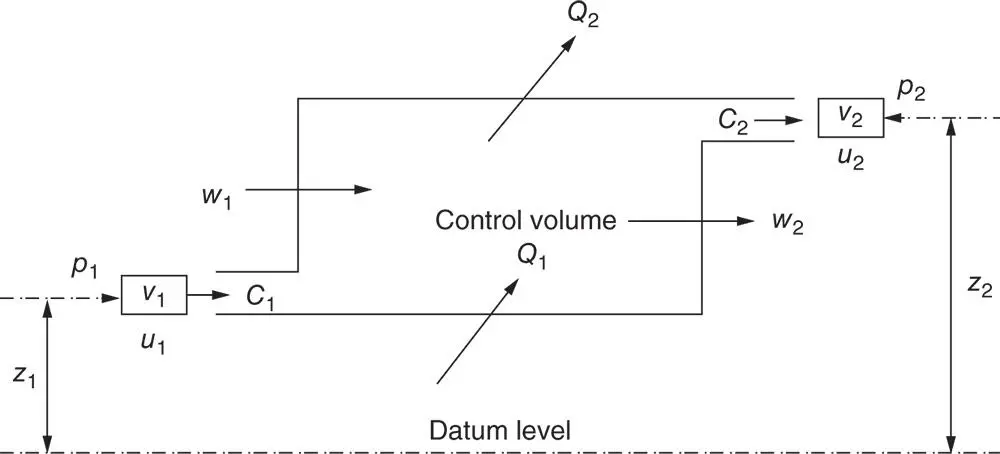
Figure 1.10Steady‐state, steady‐flow control volume.
From the law of conservation of energy, the net energy input should be equal to the net energy output plus the energy that accumulates in the control volume. For properties per unit mass (specific properties denoted by lowercase letters) and consistent energy units ( J ) for all terms, we can write
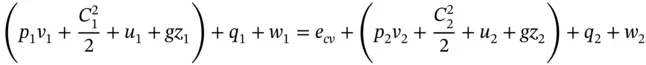
Energy is not usually allowed to accumulate in the control volume of practical thermal power plants operating on thermodynamic cycles, and therefore the term e cvwill be henceforward ignored and the energy equation is reduced to
Читать дальше