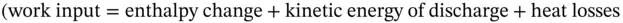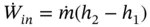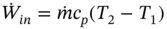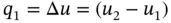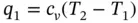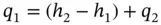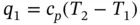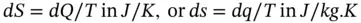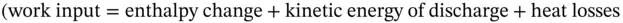
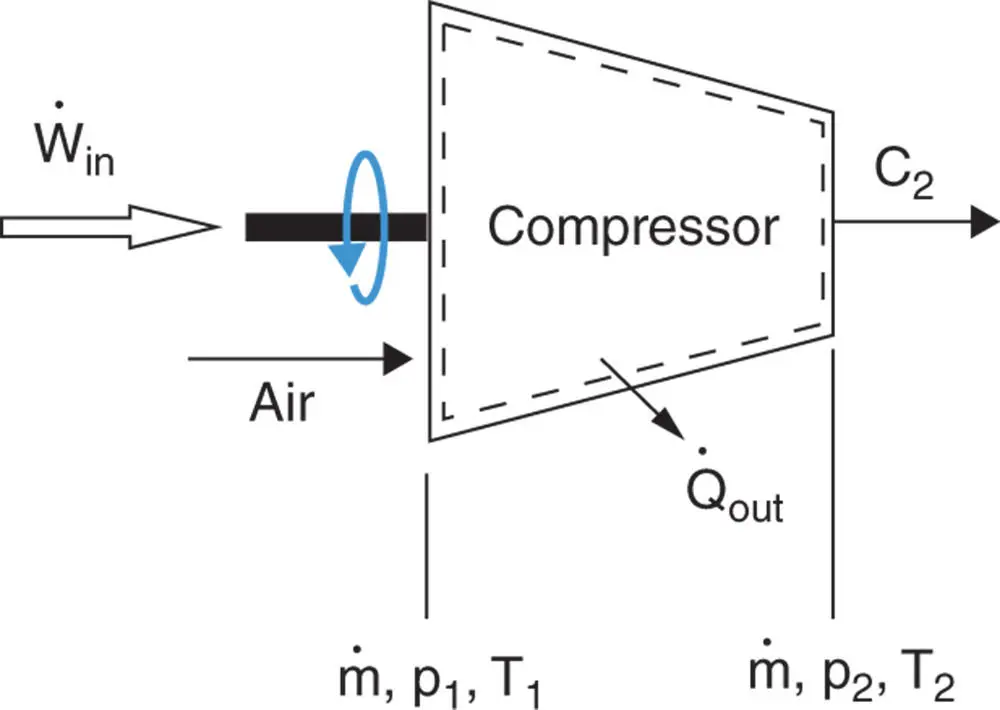
Figure 1.14Schematic diagram of air compressor.
If the velocity of the gas is reduced at the exit from the compressor so that the kinetic energy of discharge is negligible and there is no appreciable heat loss, Eq. (1.82) is reduced to
(1.83) 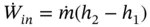
For a perfect gas, Eq. (1.83) can be written as
(1.84) 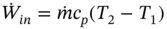
Heating gas at constant pressure or constant volume . When a gas is heated at constant volume without work or heat transfer, Eq. (1.58) is written as
(1.85a) 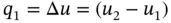
For a perfect gas,
(1.85b) 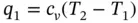
When the gas is heated at constant pressure under steady flow conditions, Eq. (1.60) is reduced to
(1.86) 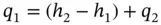
For a perfect gas with negligible heat losses, q 2= 0 and Eq. (1.86) becomes
(1.87) 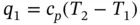
1.3.6 Second Law of Thermodynamics
The first law of thermodynamics states that energy cannot be created or destroyed but it can be converted from one form to another; and when heat is converted to work, the latter can never be greater than the former. However, it does not state how much of the heat energy, for example, can be converted to work and how efficiently. The second law, in its various statements, gives the answers to these questions. A clear statement of the second law (Rogers and Mayhew, 1992) that is relevant to the subject matter of this book and based on Planck's statement is as follows:
“It is impossible to construct a system which will operate as a cycle, extract heat from a reservoir and do an equivalent amount of work on the surroundings.”
It follows that part of the extracted heat must be rejected to another reservoir at a lower temperature. Two cases can be identified:
Heat transfer will occur down a temperature gradient as heat from high‐temperature source, such as combustion chamber in a gas turbine, is partly converted to mechanical work with the balance rejected to a low‐temperature reservoir (sink) such as the atmosphere ( Figure 1.15a). This system is known as a heat engine.
Heat can be transferred from a low‐temperature source, such as the cooling compartment in a refrigerator, up a temperature gradient to a high‐temperature reservoir (sink), such as the kitchen, with the assistance of external mechanical work ( Figure 1.15b). This system is known as a heat pump, air conditioner, or refrigerator.
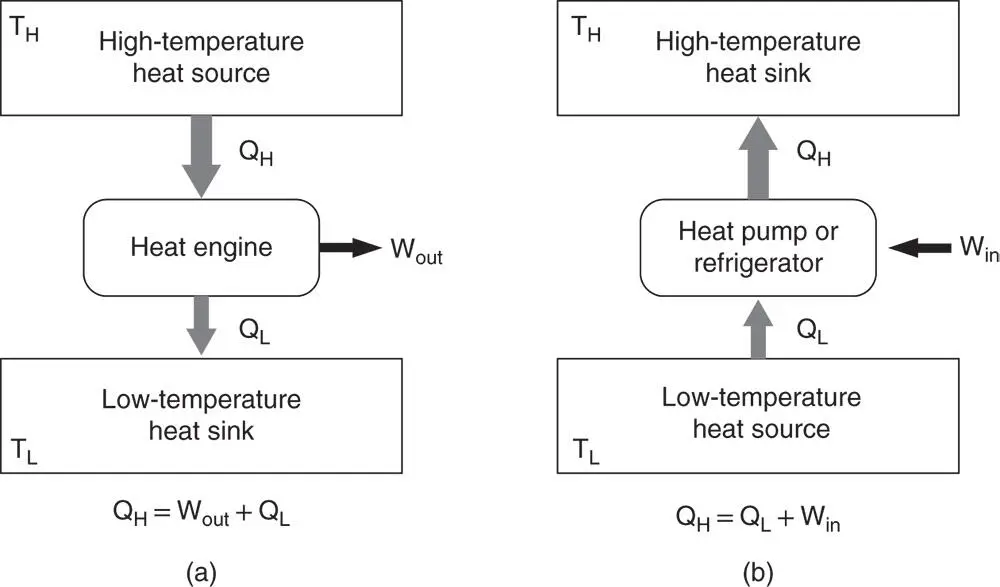
Figure 1.15Schematic arrangements of a (a) heat engine; (b) heat pump or refrigerator.
The second law of thermodynamics is also stated as the law of degradation of energy whereby the quantity of energy is conserved, but its quality (the potential to produce useful work) is not. Every time energy changes form or is transferred from one system to another, its potential to produce useful work is reduced irreversibly forever. It is then said that energy has degraded .
This law is the reason we may face an energy and/or climate crisis. All the energy that we use ultimately ends up as waste heat transferred to the earth's atmosphere and then to space.
Entropy is a thermodynamic property that is a measure of process irreversibility or energy degradation and is defined as
(1.88) 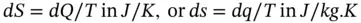
where
dS : total entropy change
ds : specific entropy change
dQ : heat transferred reversibly
T : absolute temperature at which heat is transferred
If heat is added to a system, ds will be positive (entropy increases).
If heat is removed from a system, ds will be negative (entropy decreases)
If ds = 0 during a process, the process is isentropic. The frictionless adiabatic process is an isentropic process.
A reversible process occurs when both the system and the surroundings are returned to their original conditions after the process and reverse process have been carried out. Processes in nature are irreversible, however, because reversal always causes some change to occur in the system and/or surroundings. Factors causing irreversibility include:
Friction
Unrestricted expansion
Heat transfer through a finite temperature difference
Mixing of two different gases
Chemical reactions
1.3.7 The Carnot Principle
Nicolas Sadi Carnot (1796–1832) was a French engineer who made significant contributions to the science of thermodynamics by recognising that heat engines must operate with cyclic processes. A cycle occurs when a thermodynamic system, having undergone a series of processes, arrives at a final state that is exactly the same as its initial state. In Carnot's own words (Sandfort, 1964): ‘The thermal agency by which mechanical effect may be obtained is the transference of heat from one body to another at a lower temperature’. Carnot also investigated the problem of determining the maximum work that can be extracted from the transfer of heat from high to low temperature. He eventually came up with a definition of a perfect thermodynamic engine as follows: ‘Whatever amount of mechanical effect it can derive from a certain thermal agency, if an equal amount be spent in working it backwards, an equal reverse thermal effect will be produced’. Such an engine has come to be known as the reversible engine , and the quotation as the Carnot principle . Furthermore, Carnot stated that the maximum limits of temperature between which any actual heat engine can work are the temperature of combustion of fuel and the temperature of the coldest body we can easily find and use in nature, usually the water in rivers and lakes. Figure 1.15a is the Carnot engine, and the Carnot cycle is shown in Figure 1.16in p − V and T − s coordinates:
Process 1–2: Isothermal expansion (pV = const.) with heat addition
Process 2–3: Reversible adiabatic expansion (pVγ = const)
Process 3–4: Isothermal compression (pV = const) with heat rejection
Process 4–1: Reversible adiabatic compression (pVγ = const)
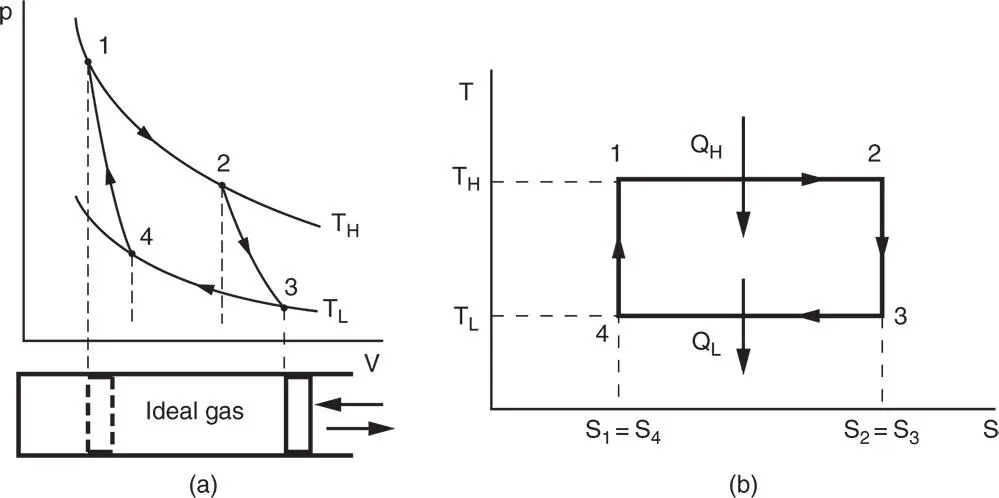
Figure 1.16Ideal Carnot engine cycle in (a) p‐V and (b) T‐s coordinate systems.
Читать дальше