This book covers both CO 2capture technologies as well as power generation technologies. These are strongly coupled when capturing CO 2from power plants. CO 2capture technologies have many of the same components found in various chemical plants, while power generation technologies is a different world where power engineering and mechanical engineering rule the ground. CO 2capture makes it necessary to deal with these areas simultaneously, with a communication between a more diversified group of engineers and scientists that is common today. It is even more complex than that because CO 2capture is also strongly coupled to transport and storage of the CO 2, which brings geologists and reservoir engineers into the game. Another aspect of this is that if CO 2capture and storage make sense, it needs to be done on a very large scale – with big implications to not only the energy industry but also to the society in general. This makes it necessary to have the economists and social sciences taking part in the development of CO 2capture and storage technologies.
Note that biomass as fuel and Bio-CO 2capture and storage (CCS) are not included in the book. Although many of the same aspects on power plant technologies and CO 2capture as for fossil fuels also apply to biomass, we believe that this subject deserves a separate treatment together with other potential negative CO 2emission technologies.
Both authors have a mechanical engineering background. Olav Bolland was active in the CCS field from the late 1980s until 2017 and has led and participated in many national and European projects within CCS. Lars Nord worked in the power generation industry for seven years before returning to academia in 2006 to pursue a PhD degree within CCS under Bolland's supervision. This work has continued until now. Both Bolland and Nord are working at NTNU – The Norwegian University of Science and Technology in the Faculty of Engineering.
Some notes on terminology in the book are as follows:
The commonly used term oxy-fuel (or oxyfuel) is not used in this book because of the misconception of having an oxidised fuel, like nitromethane (CH3NO2). Instead, the term oxy-combustion is used, which the authors believe describes the combustion in an oxygen-enriched environment more accurately. The term oxy-fuel combustion has also been extensively used in the literature.
The off-gas coming from a power plant, engine, or any other type of combustion device is sometimes referred to as flue gas and other times as exhaust gas. The latter is mostly used for gas turbines and other types of engines, while the former is mostly used for combustion plants like a coal-fired power plant or any type of furnace. Flue gas is the term used in this book with a few exceptions.
With hopes of a good read for you!
Lars O. Nord and Olav Bolland Trondheim, Norway
8 November 2019
We convey our thanks to many persons that in one way or another have been involved in the book.
Thanks to Olav Kaarstad for inputs to CO 2storage, Kjell Erik Rian for gas compression and separation, Ivar Ertesvåg for efficiency definitions and separation work, and Tord Ursin for efficiency calculations and definitions.
Multiple experts have provided inputs and feedback on the CO 2capture methods including Hallvard Svendsen for gas absorption, Konrad Eichhorn Colombo for inputs on membranes and the AZEP, May-Britt Hägg on membranes, Giorgia Mondino on adsorption, Ed Blekkan on gas reforming, Ola Maurstad on coal gasification, and Peter Koch on combustion. Thank you all!
For proofreading, we thank Zeinab Amrollahi, Aldo Bischi, and Rahul Anantharaman.
Many thanks to Konstantinos Kyprianidis and the SOFIA research group at Mälardalen University for hosting Lars Nord during his sabbatical under which the book was finalized.
And of course, last but not the least, we thank our wives Synnøve (Olav) and Nataša (Lars) for the patience and having to hear more than you probably have liked to about this book.
Nomenclature
Latin Symbols
| a |
effective interfacial area per unit volume of packing |
m 2/m 3 |
| A |
cross-sectional area |
m 2 |
| A m |
membrane surface area |
m 2 |
| c p |
specific heat capacity at constant pressure |
kJ/(kg K) |
| c v |
specific heat capacity at constant volume |
kJ/(kg K) |
| d g |
abundance of gas component g |
kg |
| F |
Faraday constant |
— |
| h |
specific enthalpy on mass basis |
kJ/kg |
 |
specific enthalpy on molar basis |
kJ/kmol |
 |
specific enthalpy of formation on molar basis |
kJ/kmol |
| H |
enthalpy |
kJ |
| H 0 |
enthalpy of formation |
kJ |
| He |
Henry's law constant |
Pa/kmol fraction gas in liquid |
| He ′ |
Henry's law constant |
(Pa m 3)/kmol gas in liquid |
| HHV |
higher heating value |
kJ/kg |
| HR |
heat rate |
kJ/kWh |
| J i |
flux through a membrane |
m/s |
| LHV |
lower heating value |
kJ/kg |
| m |
mass |
kg or t |
 |
mass flow rate |
kg/s |
| MW |
molecular weight |
kg/kmol |
| n |
polytropic coefficient |
— |
| n i |
number of moles for specie i |
mol |
| Nu |
Nusselt number |
|
| p |
absolute pressure |
Pa, bar |
| p f |
partial pressure of the permeated gas at the feed side of a membrane |
Pa, bar |
| p i |
partial pressure of component i |
Pa, bar |
| p p |
partial pressure of the permeated gas at the permeate side of a membrane |
Pa, bar |
| P |
membrane permeability |
m 3(STP)/(m h bar) |
| Pr |
Prandtl number |
|
| q i |
gas adsorbed per unit mass of adsorbent |
mol gas/kg adsorbent |
| q p,i |
volumetric flow rate through membrane |
m 3(STP)/h |
| Q |
heat |
kJ |
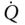 |
heat transfer rate |
kW |
| r |
radiative forcing |
W/(kg m 2) |
| R |
specific gas constant |
kJ/(kg K) |
| R u |
universal gas constant |
kJ/(kmol K) |
| Re |
Reynolds number |
|
| s |
specific entropy on mass basis |
kJ/(kg K) |
| S |
entropy |
kJ/K |
| S ads |
adsorption selectivity |
— |
| t |
time |
s or h |
| t m |
membrane thickness |
m |
| T |
temperature |
°C or K |
| u |
absolute velocity |
m/s |
| U |
internal energy |
kJ |
| v |
specific volume on mass basis |
m 3/kg |
| V |
volume |
m 3 |
 |
volumetric flow rate |
m 3/s |
| w |
specific work on mass basis |
kJ/kg |
 |
specific work on molar basis |
kJ/kmol |
| W |
work |
kJ |
 |
power |
kW |
| x |
vapour quality |
kg vapour/kg vapour+liquid |
| x i |
mole fraction in liquid phase for component i |
— |
| y i |
mole fraction in gas phase for component i |
— |
| Z |
compressibility factor |
— |
Читать дальше