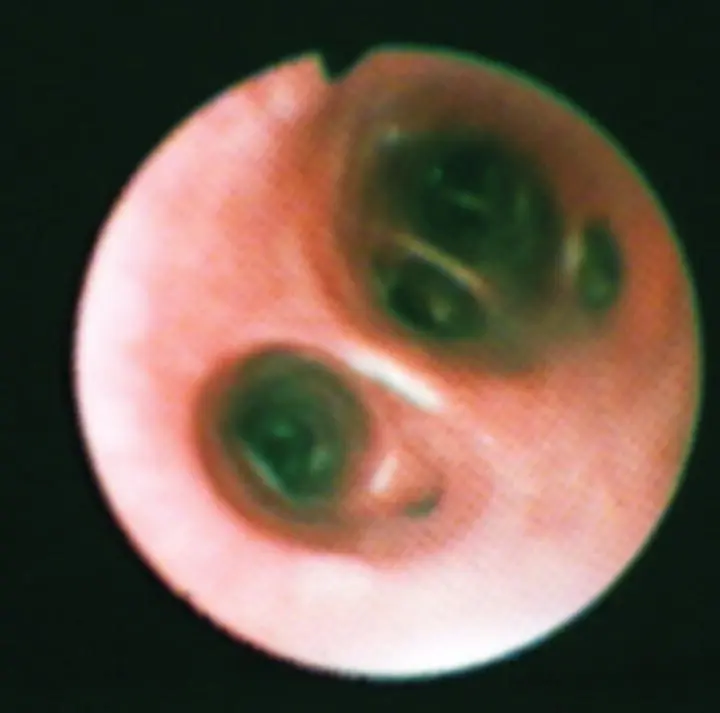
Figure 2.21 Distal airway openings in the dog exhibit relatively sharp bifurcations into round to oval airway openings. The epithelial surface is smooth.
After examining all visible airways, the scope is withdrawn from the airways, the outside is wiped with saline‐soaked gauze pads, and the biopsy channel is flushed with sterile saline. On the second entry to the airway, the scope should be kept in the center of the airways to limit upper airway and mucosal contamination when approaching the site for lavage. Success in achieving a diagnostic BAL will depend on the ability to wedge the bronchoscope gently into a small airway and isolate a segment of alveolar volume ( Figure 2.22). The goal is to flood this small wedge of lung with sterile non‐bacteriostatic saline, float the resident inflammatory cells, and gently aspirate back the fluid. Fluid should be fully ejected from the scope into the alveolar space by flushing gently with a small volume of air (3–5 ml) before beginning the aspiration. The volume of fluid used depends on the size of the scope in relation to the size of the animal, experience of the operator, underlying disease process, and degree of respiratory compromise ( Table 2.4), although ~1 ml/kg has been also recommended. If the fluid has been in contact with the alveolar space, it will be foamy because of the presence of surfactant. Flocculent material is usually mucus from bronchial contamination. If a sufficient sample is not recovered from the lavage (generally 50–70% of the fluid will be recovered), a second lavage should be performed at the same site. In human medicine, multiple lavages are commonly obtained and the first is not analyzed, because it represents more of the bronchial component rather than alveolar. It is unclear how often this is done in veterinary medicine. It is recommended to lavage at least two distinct lung sites because of inherent differences in the inflammatory cell populations among the lung lobes and with different disease processes.
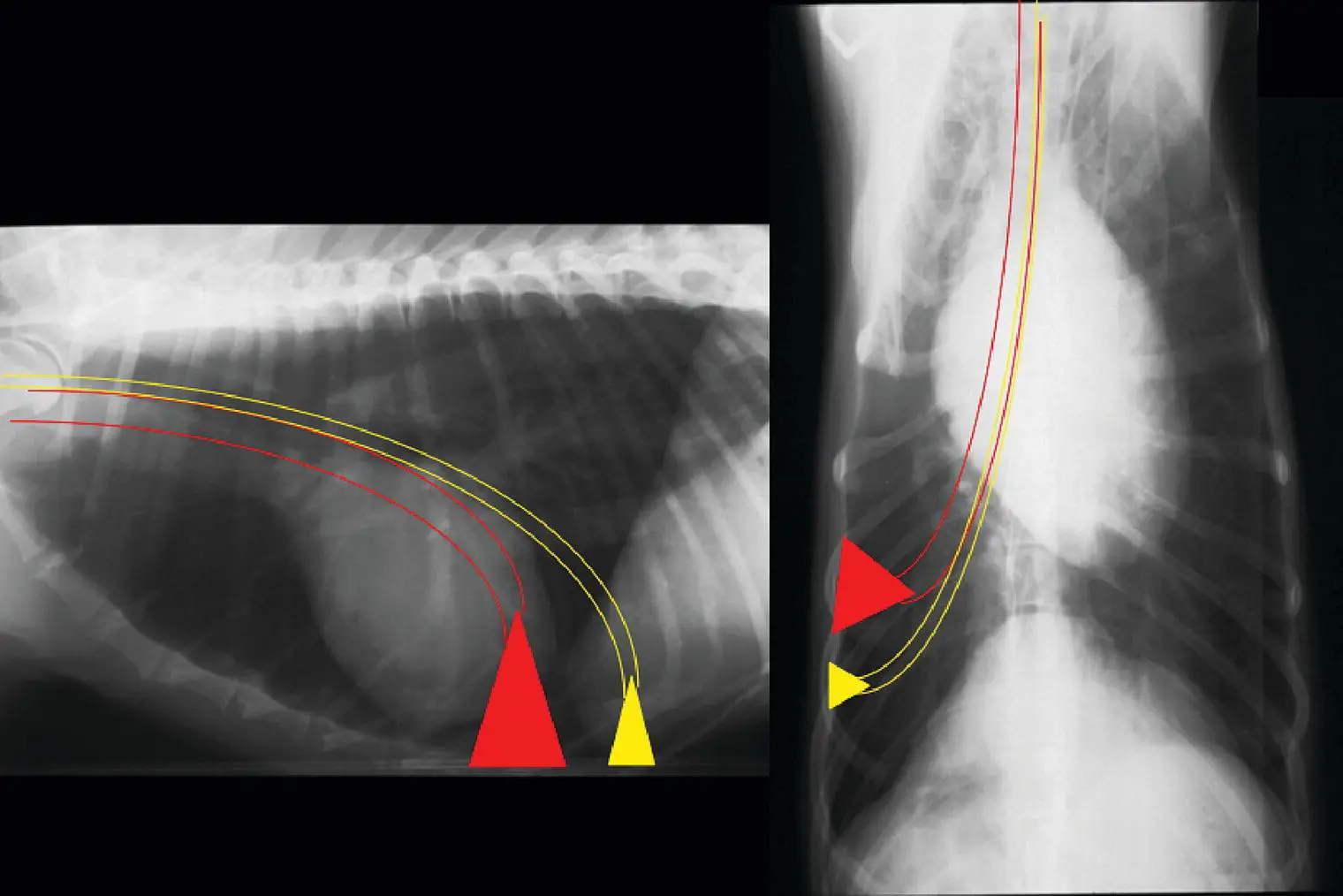
Figure 2.22 Bronchoalveolar lavage (BAL) is performed by gently wedging the bronchoscope into the smallest airway possible. The volume of the region lavaged (represented by red and yellow triangles) will depend on the size of the scope and the size of the animal.
Table 2.4 General guidelines for performing bronchoalveolar lavage (BAL).
| Scope size |
Animal size |
Milliliters per aliquot |
| 2.8–3.8 mm outer diameter |
Cats Dogs < 6 kg |
3–5 ml 5–10 ml |
| 4.9–5.5 mm outer diameter |
Dogs |
15–20 ml |
When using a scope > 6 mm outer diameter, consider passing sterile polypropylene tubing through the biopsy channel to wedge for BAL rather than using only the channel. Beware that the tubing can puncture the airway if extended with force.
Thoracocentesis is performed as a diagnostic and therapeutic technique. It can be done before radiographs are performed when physical examination suggests a pleural disorder and the animal is in distress, after thoracic ultrasound demonstrates pleural disease, or after radiographs when pleural space disease is confirmed. The region of the seventh to ninth intercostal space is clipped and scrubbed in the ventral one‐third of the chest for fluid and in the dorsal one‐third for air. A 20 or 22 gauge butterfly needle is adequate for use in small dogs and cats when small pleural effusion or mild pneumothorax is present. A fenestrated 14–18 gauge catheter with extension set works well for larger dogs and can allow relatively rapid removal of large pleural effusions. Prior to entering the chest, an extension set, three‐way stopcock, and syringe should be assembled and ready for use to limit introduction of air into the pleural space after penetration with the needle or catheter. A large bowl, ethylenediaminetetraacetic acid (EDTA) and red‐top tubes, and a culturette swab should also be readily available to allow efficient specimen collection. In some animals, sedation is needed to perform a chest tap safely. Whenever possible, it is useful to have three people available to perform a chest tap.
A sterile preparation of the lateral thorax is completed and a site on the chest wall is chosen. The needle or catheter is advanced through tented skin and then walked off the cranial border of the rib to penetrate the pleural space at a perpendicular angle. This will avoid the vessels and nerves lying along the caudal rib margin. As soon as the pleura has been penetrated, the needle or catheter is directed downward to avoid injury to the lung parenchyma ( Figure 2.23). The needle stylet is held stationary while catheter is advanced over the needle into the pleural space. When the catheter is fully in the chest, the needle is withdrawn, and the extension set is rapidly attached.
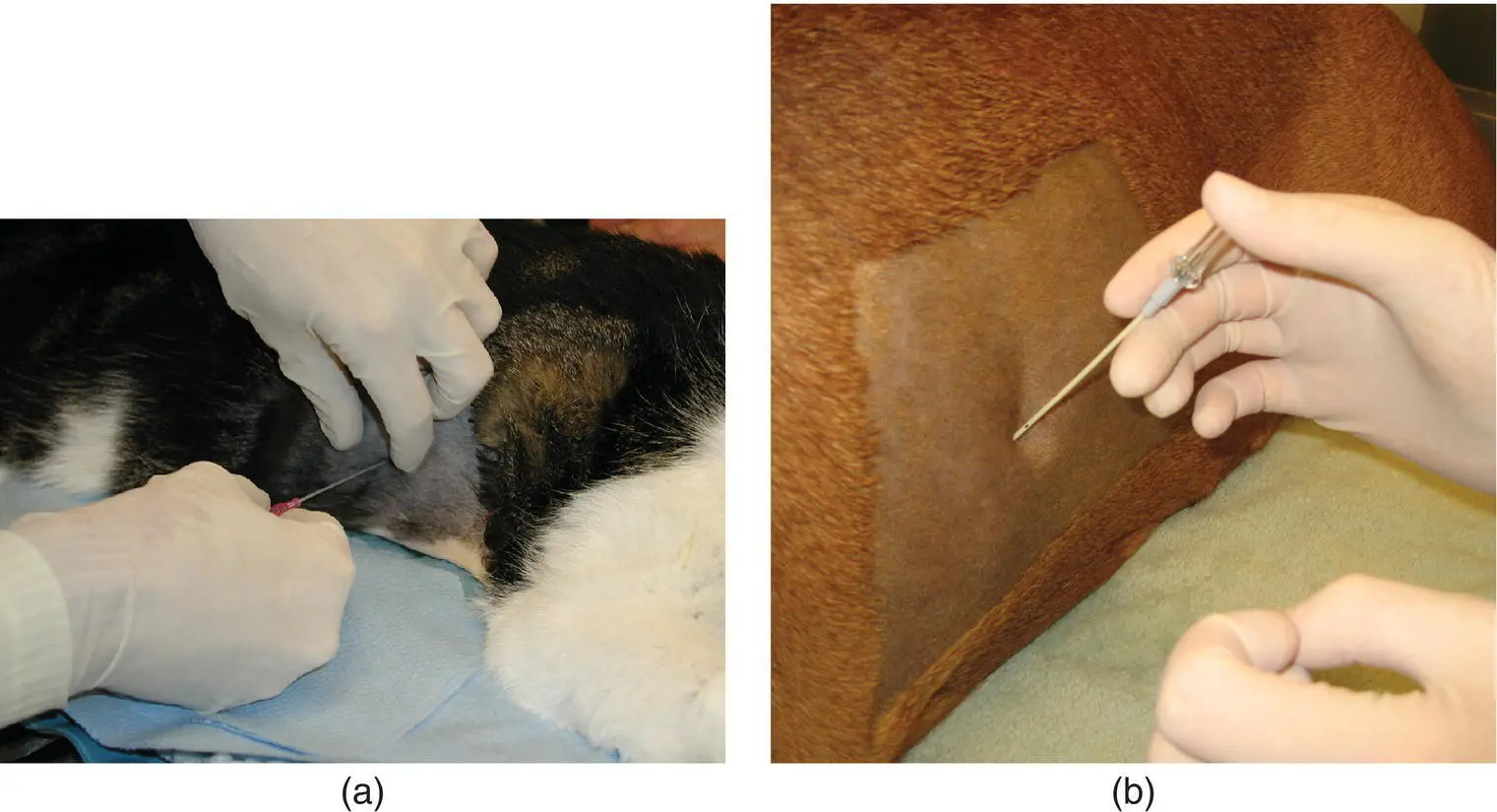
Figure 2.23 (a) To initiate thoracocentesis, the skin is tented and the catheter with needle is inserted perpendicularly between the rib spaces. (b) After the pleural space has been entered, the needle is directed ventrally and the catheter is advanced fully into the chest while the needle is held stationary.
Fine‐Needle Lung Aspiration and Biopsy
FNA of the lung is a suitable technique for evaluating diffuse interstitial or alveolar disorders or peripheral mass lesions within the thorax. It can be performed as a blind technique or with ultrasound guidance. However, because it can be associated with pneumothorax or hemothorax, careful patient selection is advised to limit complications. In animals that are not severely tachypneic or hyperpneic, percutaneous FNA of the lung can be performed safely and with minimal sedation. A small region of the chest is shaved and minor surgical preparation is completed. A 23–27 gauge 0.75–1.5 in. needle attached to a 3 ml syringe filled with air is passed perpendicularly through the skin and subcutaneous tissue cranial to the border of the rib into the lung parenchyma. It is inserted back and forth gently and quickly; then the needle and syringe are removed together for preparation of cytologic specimens. The syringe is detached from the needle and filled with air, and contents of the needle hub are gently sprayed onto a slide or cover slip for cytologic examination and Gram staining, if possible. If aspiration with an empty syringe fails to dispel material onto the slide, the syringe can be filled with 0.5–1 ml of sterile saline, and the aspirated material is suspended in the saline for a cytospin preparation. Even when the sample appears to be of low cellularity, cytopathology is recommended to detect cellular atypia or inflammation.
Figure 2.24 Ultrasound guidance is used to obtain a lung biopsy using a Temno biopsy needle.
Percutaneous lung biopsy can be obtained with use of an ultrasound‐guided biopsy needle in the anesthetized patient. This is performed more commonly in dogs than in cats. A surgical preparation is performed, and a 16–18 gauge Temno™ biopsy needle (Merit Medical Systems, South Jordan, UT) is guided into the lesion to obtain a 2 cm core tissue sample for histopathology ( Figure 2.24). After either aspiration or biopsy, the animal should be placed in lateral recumbency with the side of the aspiration facing downward for 15–30 minutes to promote the development of a clot or seal at the aspiration site. Typically, an ultrasound is performed after the procedure to screen for hemorrhage or pneumothorax. Visualization of the normal “glide” sign as the lung slides across the pleura rules out one of these complications. An increase in respiratory rate or effort or detection of absent lung sounds near the site of aspiration would indicate hemorrhage or pneumothorax and the need for intervention.
Читать дальше
















