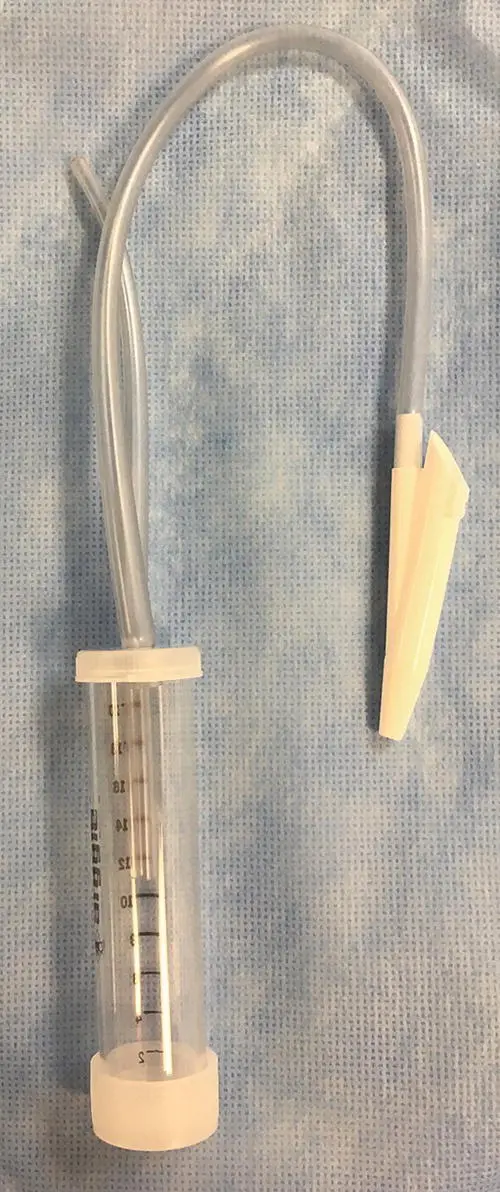
Figure 2.5 A suction trap can be secured below the patient during the tracheal wash. One tube is connected to the three‐way stopcock and catheter used for the tracheal wash, while the other is connected to a suction device.
Non‐bronchoscopic BAL has also been reported in the cat, and the cell distribution obtained on cytology matches that found with bronchoscopy (Hawkins et al. 1994). For this procedure, the cat is anesthetized and intubated with a sterile endotracheal tube similar to the method used for a transoral tracheal wash. The cuff is inflated and the cat is placed in lateral recumbency with the most affected side down. Aliquots of warmed sterile 0.9% saline (5 ml/kg, 1–3 aliquots) are instilled directly into the endotracheal tube using a 35 ml syringe with syringe adapter. Fluid is retrieved by hand aspiration. Elevating the hindquarters can facilitate collection, and approximately 65–70% fluid retrieval should be expected. Alternately, a urinary catheter (6–8 French) can be passed gently through a sterile endotracheal tube until resistance is met (Foster et al. 2004), in a manner similar to that employed when a modified stomach tube is used to perform blind BAL in a dog. Instillation and aspiration of 5–10 ml of sterile saline provide an adequate lavage sample for cultures and cytology. With either procedure, respiratory rate and pulse oximetry should be monitored to detect untoward reactions and oxygen supplied as needed. In cats, pre‐treatment with terbutaline (0.01 mg/kg subcutaneously) is recommended prior to any airway procedure.
Transtracheal wash is appropriate for larger dogs ( > 8 kg) or those that cannot be anesthetized for a transoral tracheal wash (e.g. an older dog with laryngeal paralysis). Generally only local anesthesia is needed, although mild sedation with acepromazine and butorphanol or a similar short‐acting combination can facilitate completion of the procedure. The animal is in a sitting or standing position with the head held upward and the neck gently extended. Over‐extension of the neck is uncomfortable for the patient and could flatten or tense the trachea, making the procedure more difficult.
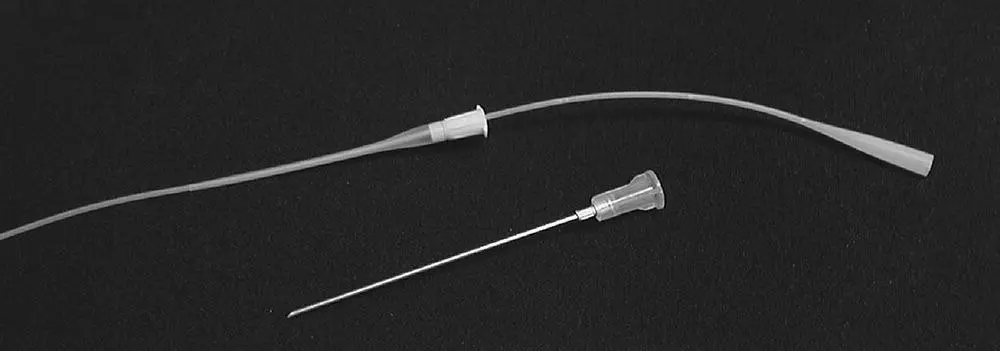
Figure 2.6 An over‐the‐needle catheter can be used as a cannula to perform a transtracheal wash using a sterile urinary catheter.
The easiest way to perform a transtracheal wash is to use a 14 or 16 gauge over‐the‐needle catheter as a cannula to penetrate the trachea and a long, sterile 3.5 French urinary catheter to pass down into the airways for sample collection ( Figure 2.6). Ensure that the long catheter will pass readily through the short one before starting the procedure. The trachea can be entered at the cricothyroid notch, but it is preferable to enter the trachea lower on the neck between the tracheal rings to avoid potential damage to laryngeal structures ( Figure 2.7a). This will also facilitate collection of a sample from more distal airways. Choose a site on the neck where the trachea is easily palpated and can be stabilized against the neck muscles for insertion of the catheter. The ventral portion of the neck is clipped and lightly scrubbed with antiseptic solution followed by alcohol wipes. Local anesthesia with lidocaine (0.25–0.5 ml) is applied at the skin and subcutaneous tissue down to the level of the tracheal rings. A more complete surgical preparation is performed after local anesthesia is instilled.
To begin catheter placement, the skin is tented at the site of lidocaine infusion and the catheter is passed through the skin at a perpendicular angle to the neck, with the bevel of the needle facing downward. If needed, a small stab incision can be made in the skin to facilitate passage of the catheter. When preparing to enter between the tracheal rings, the trachea is firmly stabilized against the neck muscles to prevent it from moving away from the needle. The tracheal rings and annular ligament can often be felt with the tip of the needle, allowing entry directly between the cartilage rings. The trachea is almost directly below the skin and only a minor amount of forward motion is required to enter the lumen. A distinct pop is usually felt when the needle enters the tracheal lumen, and the dog often coughs. With the needle in the airway, the angle between the needle or catheter and the trachea is decreased to 60° to facilitate passage of the catheter down the center of the airway ( Figure 2.7b). The needle is withdrawn at this stage to pass the sampling catheter through the short catheter to the level of the carina ( Figure 2.8).
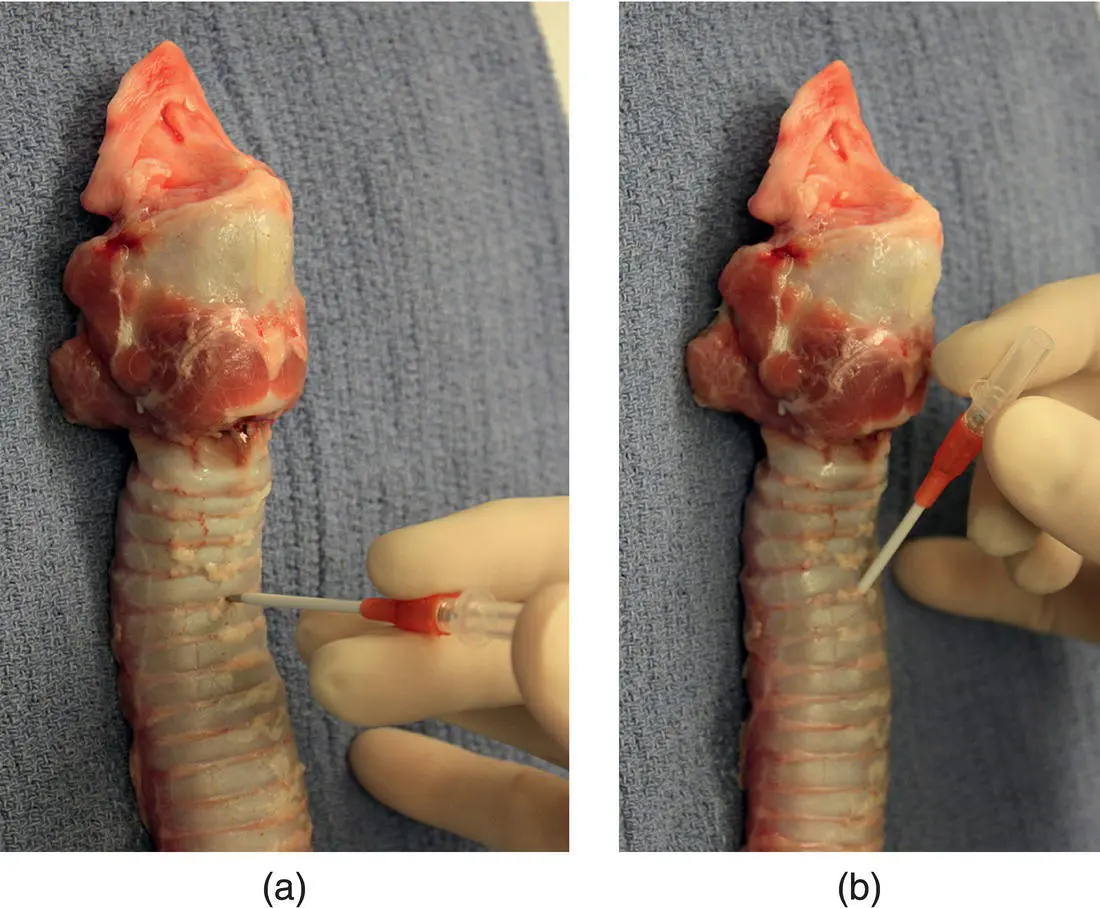
Figure 2.7 (a) To initiate a transtracheal wash, the needle‐through‐catheter is directed into the trachea at a perpendicular angle. (b) After it has penetrated between the tracheal rings, the hub of the needle‐through‐catheter is moved toward the neck to create a more parallel angle to feed the catheter off the needle in preparation for passing the urinary catheter.
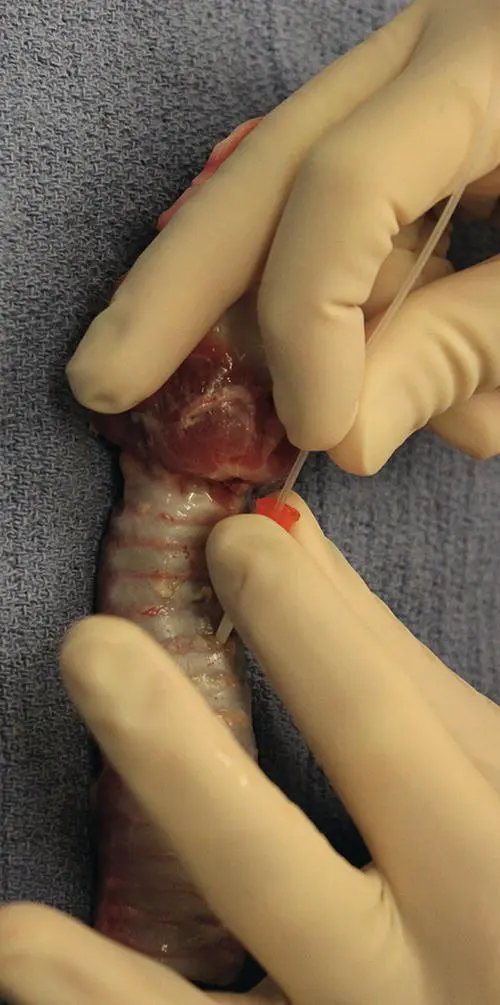
Figure 2.8 The short catheter has been seated between the tracheal rings and is grasped firmly while the long catheter is passed down the trachea for collection of an airway sample.
The urinary catheter should move freely down the airway and passage usually stimulates coughing. If neither of these occurs, the sampling catheter could be traveling in subcutaneous tissue and both catheters should be removed in order to repeat the approach to the trachea.
When the sampling catheter is fully deployed, the three‐way stopcock and syringe can be attached to perform the airway wash. Individual aliquots of saline (4–10 ml) followed by 2–3 ml of air are instilled into the airway, and suction is used to retrieve the fluid and cells from the lower airway, as detailed earlier.
Respiratory Endoscopy
Rhinoscopy
A full rhinoscopic examination includes evaluation of the caudal nasopharynx to visualize nasal openings at the choanae and inspection of the rostral nasal cavity. The best equipment to use in evaluating the caudal nasopharynx is an endoscope capable of 180° flexion that is ~5 mm in outer diameter ( Figure 2.9). Smaller flexible endoscopes can be used, but are at greater risk for damage if the animal gags and the pharynx closes down on the scope. Examining the nasopharynx is a highly stimulatory procedure. Local anesthesia of the oropharynx with lidocaine gel on long cotton swabs can facilitate placement of equipment around the soft palate. Two mouth gags are in place to hold the mouth wide open during placement of the endoscope or other equipment. Caution is warranted when performing this examination in cats, because over‐extension of the jaw can reduce retinal blood flow through the maxillary artery (the continuation of the external carotid as it crosses the mandible), resulting in blindness (Barton‐Lamb et al. 2013). Therefore, excess opening of the jaw should be avoided and time spent completing the examination kept to a minimum. Extending the neck and pulling the tongue forward will also help place the instruments in the proper position. To achieve the appropriate view with an endoscope, the non‐flexed scope is passed into the oral cavity beyond the soft palate and then flexed until the light can be seen above the soft palate. The scope is then pulled forward (with the flex in place) until the choanae are visualized. Note that the image is upside down and backward ( Figure 2.10).
Читать дальше
















