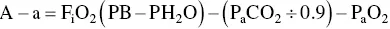1 ...8 9 10 12 13 14 ...19 Table 2.1 Normal blood gas values for dogs and cats.
|
Dog |
Cat |
| P aO 2(mmHg) |
90 (80–105) |
100 (95–105) |
| P aCO 2(mmHg) |
37 (32–43) |
31 (26–36) |
| pH |
7.35–7.45 |
7.35–7.45 |
| HCO 3(mmol/l) |
22 (18–26) |
18 (14–22) |
HCO 3, bicarbonate; P aCO 2, partial pressure of carbon dioxide; P aO 2, partial pressure of oxygen.
Alveolar–Arterial Oxygen Gradient and PF Ratio
The alveolar–arterial (A–a) oxygen gradient estimates the difference between the calculated alveolar oxygen level expected for the animal and the measured arterial oxygen level. Thus, the A–a gradient corrects for the level of ventilation performed by the animal and allows comparison of blood gas data through the course of disease that is not impacted by the effect of an increase or a decrease in P aCO 2on P aO 2. The A–a oxygen gradient is calculated as:
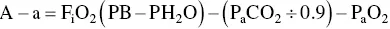
where F iO 2is the fraction of inspired oxygen (0.21 on room air), PB is the barometric pressure (in mmHg), PH 2O is the water vapor pressure (47 mmHg at 37 °C), and R is the respiratory quotient (ratio of CO 2production to O 2consumption, usually assigned a value between 0.8 and 1.0). P aO 2and P aCO 2are obtained from blood gas analysis. Normal value for the A–a oxygen gradient is < 15.
The PaO 2/FiO 2ratio (PF or oxygenation ratio) provides a measure of the ability of the lung to oxygenate as the fraction of inspired oxygen changes from room air to 100% oxygen. This is calculated by dividing arterial oxygen by FiO 2(ranging from 0.21 to 1.0). Normal animals have a PF ratio of > 500 at sea level. Values between 300 and 500 indicate mild impairment of oxygenation, while values < 200 indicate serious decrements in oxygenation. A PF ratio < 200–300 is one of the criteria for a diagnosis of acute respiratory distress syndrome.
Obtaining an arterial blood gas, calculating the A–a gradient, and assessing response of hemoglobin saturation or P aO 2to exogenous oxygen supplementation allow assumptions to be made about the most likely mechanism causing hypoxemia ( Table 2.2). This can help determine the most likely cause of hypoxemia, although ventilation/perfusion mismatch underlies the pathophysiology of hypoxemia in almost all lung diseases, and many clinical disorders have multiple contributors to hypoxemia.
Diagnostic Imaging
Radiography
Radiography is often the key to creating an appropriate list of differential diagnoses for the respiratory case and for determining the type of sampling method that is most likely to achieve a final diagnosis, such as endoscopy, fine‐needle aspiration (FNA), or a tracheal wash ( Table 2.3). It will also help determine the need for advanced imaging, including fluoroscopy, ultrasound, nuclear scintigraphy, or computed tomography (CT). The widespread use of digital radiography has enhanced the evaluation of pulmonary patterns, although overlying structures can still confuse interpretation. Specific features of these tests are presented in the relevant disease sections.
Table 2.2 Respiratory causes of hypoxemia.
| Mechanism |
Clinical attributes |
Causes |
| Hypoventilation |
High P aCO 2Normal A–a gradient Improved by oxygen supplementation Improved by increasing alveolar ventilation |
Anesthesia Upper airway obstruction Neuromuscular weakness CNS disease |
| V/Q mismatch |
Increased A–a gradient Mildly increased P aCO 2Markedly improved by oxygen supplementation |
Virtually any lung disease |
| Shunt |
Increased A–a gradient Not improved by oxygen supplementation Not improved by increasing alveolar ventilation |
Congenital right to left cardiac shunts Acute respiratory distress syndrome |
| Diffusion impairment |
Increased A–a gradient Seldom a major cause of hypoxemia at rest Causes hypoxemia during exercise or with low inspired oxygen Improved by oxygen supplementation |
Interstitial lung disease Pulmonary edema |
| Reduced inspired oxygen |
Improved by oxygen supplementation Causes hypoxemia during exercise or when diffusion is impaired |
High altitude |
A–a, alveolar–arterial; CNS, central nervous system; P aO 2, partial pressure of oxygen.
Table 2.3 Airway sampling techniques for various lung patterns.
| Radiographic pattern |
Differential diagnoses |
Sampling technique |
| Interstitial |
Viral pneumonia Rickettsial pneumonia Protozoal pneumonia Hemorrhage Vasculitis Pulmonary fibrosis Neoplasia Early pulmonary edema Aspiration pneumonia |
Fine‐needle aspirate Lung biopsy Bronchoscopy Tracheal wash |
| Bronchial |
Chronic bronchitis Feline bronchitis/asthma Bronchiectasis Parasitic bronchitis Early bronchopneumonia |
Tracheal wash Bronchoscopy |
| Alveolar |
Bronchopneumonia Aspiration pneumonia Fungal pneumonia Hemorrhage Pulmonary edema Neoplasia Non‐cardiogenic pulmonary edema |
Tracheal wash Bronchoscopy |
| Consolidation |
Neoplasia Lung lobe torsion Consolidating pneumonia Granuloma Bronchial obstruction Feline bronchitis Foreign body inhalation |
Bronchoscopy Fine‐needle aspirate Tracheal wash |
| Vascular |
Congenital heart disease Congestive heart failure Heartworm disease Pulmonary hypertension Pulmonary thromboembolism |
Echocardiography |
| Effusion |
Hydrothorax Pyothorax Hemothorax Chylothorax Neoplasia Diaphragmatic hernia |
Thoracocentesis |
Orthogonal views are always recommended for evaluation of thoracic contents, and assessment of the thorax is improved by obtaining both left and right lateral views, as well as a dorsoventral or ventrodorsal image. Lateral views provide an optimized view of the lung closest to the radiographic unit, therefore a left lateral projection would be more likely to identify infiltrates in the right middle lung lobe in a patient with aspiration pneumonia. A right lateral projection might be preferred to investigate airway collapse at the left cranial lobar bronchus (cranial and caudal segments). The dorsoventral view provides better imaging of the cardiac silhouette and pulmonary vessels, although the ventrodorsal view allows better assessment of small volumes of pleural effusion, as well as infiltrates in the ventral or lateral portions of the lung. Importantly, attempts should be made to confirm the presence of pulmonary nodules on both a lateral and an orthogonal view.
In some patients, cervical radiographs can provide valuable information on the extrathoracic respiratory tract and its potential role in thoracic disease. Loss of the nasal air column from the nasal cavity into the nasopharynx, elongation or thickening of the soft palate, the suggestion of laryngeal edema or mass, air in the laryngeal saccules, or caudal retraction of the larynx are clues to the presence of an upper airway obstructive lesion that could be contributing to disordered breathing or a lower respiratory tract process.
Ultrasound of the larynx can be used to evaluate patients for laryngeal paralysis or mass lesions, and cervical tracheal collapse can also be identified with ultrasound. However, these studies can be technically challenging because soft tissues are adjacent to air‐filled structures, which causes marked attenuation of the ultrasound beam. However, valuable information can be gained by an experienced ultrasonographer.
Читать дальше