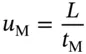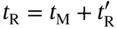The stationary phase is a liquid, and the air peak is not soluble at all. The air peak therefore moves with the carrier gas, and its retention time is a good indicator of average carrier gas velocity.
The carbon dioxide (CO2) solubility is 25 %, so an average CO2 molecule spends 75 % of the time traveling and only 25 % of the time stopped. Therefore, the CO2 peak moves 75 % of the distance that the carrier gas moves.
The propane solubility is 50 %, so an average propane molecule spends half the time traveling and half the time stopped. Therefore, the propane peak moves 50 % of the distance that the carrier gas moves.
The 1‐butene solubility is 75 %, so an average molecule spends only 25 % of the time traveling and 75 % of the time stopped. It follows that the 1‐butene peak moves only 25 % of the distance that the carrier gas moves.
These convenient values for the component solubilities are just simple examples assumed for discussion purposes, but every substance has a real solubility in a given liquid that depends only on the temperature and pressure.
Figure 3.1shows the location of each peak when the air peak has reached the end of the column and is about to flow into the detector. At that moment, the four peaks in our example are equally spaced along the column. Similar spacing is a common occurrence in real columns, but a very strange thing then happens to the chromatogram. The exercise that follows challenges you to discover what comes next.
Imagine that the effluent from the column in Figure 3.1flows into a detector and the detector signal is recorded in the form of a chromatogram. For this exercise, assume Figure 3.1shows the correct position of the four peaks in the column at three minutes after sample injection.
Now predict what the chromatogram will look like after all the peaks have exited the column.
It's worth your time to stop for a moment and try to do this; you will learn a lot from the exercise. Be careful; it's more difficult than it looks!
You can draw your chromatogram on Figure 3.2. Sketch the chromatogram you would expect to get, showing your chosen time base in minutes and the exact positions of the four peaks.
Based on the information given in Figure 3.1, draw the chromatogram you would expect to see after all four peaks have passed through the detector. Your chromatogram should show the four peaks at their correct retention times. The injection marker is time‐zero on your chromatogram, but you must decide the time scale. In this exercise, don't worry about peak widths. 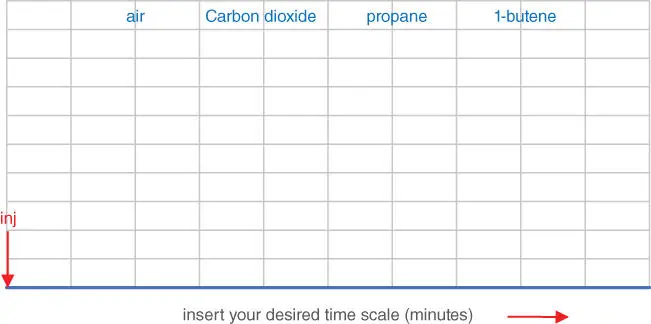
Figure 3.2Draw Your Own Chromatogram.
How confident are you of your chromatogram? People rarely get it right at first attempt. The most common mistakes are:
Inserting all four peaks between 0 and 3 mins on the chromatogram
Assuming all four peaks are equally spaced
Assuming three of the peaks are equally spaced
Assuming any peak has a fractional retention time (none do)
Remember to measure retention times from sample injection to the apex of each peak; i.e ., the retention of the average molecule.
Want to try again? We'll reveal the correct answer later.
Significance of the air peak
The air peakis a valuable indicator of column performance. Since air doesn't dissolve in column liquids to any significant extent, the air peak remains in the gas phase all the way through the column – traveling at full gas velocity. We call it an unretained peak. Its position on the chromatogram indicates the elapsed time for the carrier gas to travel from one end of the column to the other end.
If using a detector that doesn't respond to air, another unretained peak can act as a surrogate. For instance, methane often serves as an adequate “air peak” on a flame ionization detector.
Since we know the column length ( L ), the air peak retention time ( t M) allows us to calculate the average carrier gas velocity ( u M) in m/s:
(3.1) 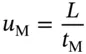
We don't need this information now, but Equation 3.1is used in more advanced work to optimize the carrier gas flow rate.
The air peak location is good to know when troubleshooting because component molecules can't travel faster than the carrier gas, so no peaks from the current sample injection can elute before the air peak.
But the air peak has a much more significant role because it shows how long the carrier gas took to pass through the column. Recall that component molecules can move along the column only when they are in the carrier gas. So, to get through the column, all component molecules must spend the same time in the carrier gas as the air peak does.
As a way of emphasizing the point, imagine that the column had no liquid inside; where would the peaks be on the chromatogram?
Yes, of course, all the peaks would elute together with the air peak. With no liquid present, no separation can occur. The air peak reveals the time that component molecules spend in the gas phase, and it's the same for every component.
As an aside, realize from this imagined experiment that the time a peak spends in the liquid phase is proportional to the amount of liquid phase present in the column. In packed columns, the liquid film can slowly evaporate into the carrier gas, thereby, over time, reducing the observed peak retention times .
On a more typical chromatogram such as the one in Figure 3.3, the component peaks come out some time after the air peak. This additional retention time, different for each component and plainly visible on the chromatogram, is the time that each component spends in the liquid phase.
An air peak tells us how long the carrier gas takes to get through the column, which is also the time that each component spends in the gas phase. The additional retention time for each peak is the time that peak spends in the liquid phase. 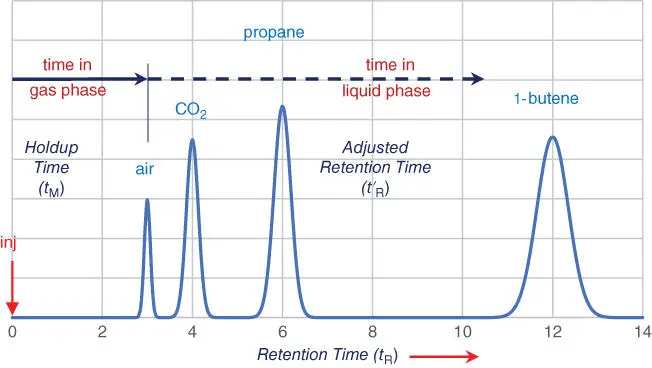
Figure 3.3Significance of an Air Peak.
Figure 3.3illustrates the point: the peak retention time ( t R) is the sum of two times; the time that the peak spends in the gas phase ( t M) plus the time it spends in the liquid phase ( t ′ R):
(3.2) 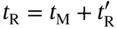
Notice that the air peak neatly divides the chromatogram into two time zones and makes them easy to measure:
From injection to the air peak is the time that all components spend in the gas phase. This time is often called the “dead time” because it doesn't contribute to separation. The formal name for the time in the gas phase is the holdup time.
From the air peak to the apex of each peak is the average time that each component spends in the liquid phase. The formal name for the time in the liquid phase is the adjusted retention time.
These chromatogram measurements are an essential prerequisite for optimizing or troubleshooting column performance. For example; they allow the optimum setting of column temperature. We plan a second book to explore these more advanced techniques.
Figure 3.3is also the correct answer for the chromatogram you were invited to draw on Figure 3.2. The four peaks are centered at 3, 4, 6, and 12 minutes from sample injection. You may find this surprising; the peaks are no longer equally spaced! Let's see how that happened.
Читать дальше