9 S9. This question requires you to make deductions from what you have learned so far.For a particular sample gas, what properties of the gas‐liquid equilibrium are necessary to produce a symmetrical peak?Select all the correct answers and none of the incorrect answers:The proportion of molecules in the gas and liquid phases must remain constant when the component concentration changes.The equilibrium must form very rapidly so more equilibria occur while the peak is in the column.The overall time the molecules spend in the gas phase must be equal to the time they spend in the liquid phase.The solubility of the sample gas must not change with its concentration.
1
1 2.1 Gases Dissolve in Liquids
2 2.2 A Different Gas
3 2.3 Forming an Equilibrium
4 2.4 The Carrier Gas Moves
5 2.5 The Second Equilibrium
6 2.6 The Third Equilibrium
7 2.7 The Fourth Equilibrium
8 2.8 The Fifth Equilibrium
9 2.9 Effect of Having More Equilibria
| 2.1 |
 |
Distribution constant |
| [ A ] M |
Equilibrium concentration of substance A in mobile phase |
| [ A ] S |
Equilibrium concentration of substance A in stationary phase |
| H |
Plate height |
| K c |
Distribution constant |
| N |
Plate number |
When first introduced, these words and phrases were in bold type. You should now know the meaning of these technical terms. If still in doubt, consult the Glossary at the end of the book:
1 distribution
2 distribution constant
3 dynamic equilibrium
4 Gaussian
5 Henry's law
6 partition
7 plate height
8 plate number
9 signal noise
10 solubility
11 solute
12 solvent
“ It's a paradox worth repeating. The different kinds of molecules that are injected into a column all travel along the column at the same speed, yet emerge from the column at different times, thus becoming separated from each other. Separation is what chromatographs do. You certainly need to know how ”.
Looking back at Figure 2.9, why is the propane peak exactly halfway along the column? Think about it.
Taking the question one stage further: What would have to happen for another peak to be at a different location in the column, separated from the propane peak?
Simple explanations of gas chromatography often say that different peaks move at different speeds and come out of the column at different times. While this is empirically true, it's a circular argument and explains nothing. It's plainly true that if the peaks come out of the column at different times they must be traveling at different average speeds, but it's illogical to then conclude that the peaks are separated because they move at different speeds. It doesn't explain anything.
A more realistic explanation
We need a better explanation. Let's start from the obvious truism that when the sample molecules are in the column, they must always be in the gas phase or the liquid phase. There's nowhere else for them to be.
And we know that the liquid phase doesn't move.
Therefore:
When the sample molecules are dissolved in the liquid phase, they are held in place and are not moving along the column.
When the sample molecules are in the gas phase, they move along the column at the same speed as the carrier gas.
Note that we refer to movement along the column. Molecules are always moving randomly in every direction, and this contributes to peak broadening, but random motion contributes nothing to positive movement along the column.
There are only two speeds along a chromatograph column; stop or go!
This is true for every peak. Every injected molecule must spend enough time in the gas phase to transit through the column. Therefore, each molecule travels at the same speed and spends the same time traveling as the other molecules do. It's not true to say that different molecules travel at different speeds.
Separation is not caused by motion at all. It's caused by stopping; the time that different molecules stay motionless in the liquid phase. The less soluble molecules don't stop for long and move quickly through the column, while the more soluble ones hang around in the liquid, slowing them down. It's that simple.
Notwithstanding the stop‐go mechanism, one might successfully argue that the peaks really are travelling at different average speeds through the column. Yes, of course, this is the overall result. But talking about the average speed of a peak obscures what is really happening in the column. Instead, chromatographers may refer to the migration rateof the peak along the column. The word “migration” infers a gradual movement by alternately stopping and going, to remind us what is really going on in there.
It should now be clear why the propane peak in Figure 2.9is exactly in the middle of the column. We assumed that 50 % of the propane molecules dissolve in the liquid phase and 50 % remain in the gas phase. Remember that those molecules are rapidly moving between the two phases. So the average propane molecule spends 50 % of its time in the gas phase moving at carrier speed and 50 % of its time in the liquid phase going nowhere. Therefore, when the carrier gas that was present during sample injection reaches the end of the column, the propane peak is exactly half‐way.
Figure 3.1applies this logic to two additional peaks, one more soluble and one less soluble than propane. The 1‐butene peak is more soluble, so we would expect it to spend more time in the liquid phase and elute from the column later than the propane. And so it does. For instance, if the solubility of 1‐butene is 75 %, only 25 % of the molecules move each time the carrier gas moves, and the peak stays in the column twice as long as the propane peak does. The carbon dioxide peak is less soluble: If its solubility is 25 %, then 75 % of its molecules move with the carrier gas.
Drawing peaks above a column is a common way to show the position of component molecules within the column at a certain instant of time. Here, an air peak (which doesn't dissolve in the liquid phase) has moved along with the carrier gas and has just arrived at the column end. At that instant, the three colored peak drawings indicate the position within the column of the other component molecules, predictable from their solubility in the liquid phase. 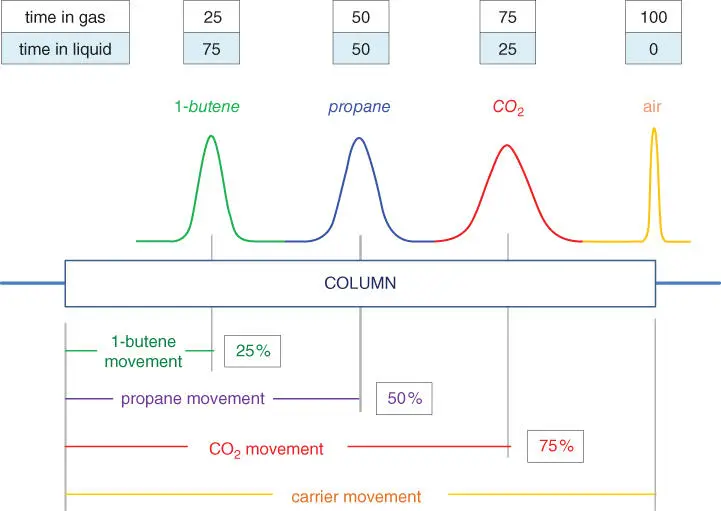
Figure 3.1Effect of Component Solubility.
This is the true cause of separation. When in the gas phase, all sample molecules move along the column at the same speed as the carrier gas. But when in the liquid phase, the molecules stop moving and the more soluble ones stop longer than the less soluble ones do .
Figure 3.1illustrates the net effect of solubility difference. It shows the location of four components peaks at the exact moment the air peak reaches the end of the column. A small white‐and‐blue equilibrium diagram indicates the solubility of each peak.
Figure 3.1assumes that:
Читать дальше














