“ What happens inside a column? What makes the peaks form? Why are peaks that funny shape? Why are some peaks wider than other peaks? These are good questions, and now is the time for answers ”.
The secret to understanding process gas chromatographs is knowing how the columns separate the components of the sample. PGC training courses often omit this important knowledge, preferring to focus instead on the mechanics and electronics of the instrument itself. It's true that special skills are required to properly set up and maintain the equipment. And you must learn those skills. Yet, even if you gain perfect knowledge of the electromechanical systems, you won't be competent with process gas chromatographs until you clearly understand what the columns are doing.
You'll need to know how a column really separates molecules of one kind from molecules of another kind. It's not sufficient (nor true) to say that some kinds of molecule move faster than others do.
You'll also need to know what determines the shape of a chromatogram peak, particularly its width. So let's look a little closer at some typical peaks.
Looking back at the chromatogram in Figure 1.7, if you examine any individual peak, it is easy to see that even identical molecules don't reach the detector at the same time. Relative to the time of the peak apex, some molecules arrive earlier, and some arrive later. Take a look at the propane peak, for instance: its base width is about 40 s, which means propane molecules start arriving at least 20 seconds before their most frequent and average time (at peak apex) and continue for at least 20 seconds after that, gradually dropping back to zero. This variation in the elution time of identical propane molecules determines the width of the propane peak and its characteristic shape.
At this point, you should be wondering why identical molecules don't spend an identical amount of time in the column. Whatever happens in there, surely identical molecules must experience identical delay and emerge from the column at the same time? No, they don't. Some emerge a little earlier, and some emerge a little later. Any useful explanation of chromatography must account for that inconvenient fact.
Of course, anything that makes a peak wider is a nuisance because it's more difficult to separate wide peaks from each other than to separate narrow peaks. So, as a practical matter, we need to know how to minimize the peak width, and that is one of the most important questions in gas chromatography! The answer will become apparent as you work through the book.
What happens inside the column
Inside the column, sample molecules that are traveling in the carrier gas touch the stationary phase. What happens next depends on what kind of stationary phase is present, a solid or a liquid.
Most PGC columns employ a liquid stationary phase, which works by selectively dissolving the component molecules. Since they are so common, it is reasonable to use liquid‐phase columns as our example for explaining how the chromatographic process works. Therefore, the rest of this chapter will discuss only gas‐liquid interaction.
Columns employing a solid stationary phase are used to separate simple gases like hydrogen, oxygen, nitrogen, or methane. Gas‐solid columns work by selectively adsorbing the sample molecules. This is a different mechanism, but it has the same effect; the column retains one kind of molecule longer than it retains another kind of molecule.
How gas and liquid interact
In a gas‐liquid column, the component molecules touch a stationary liquid phase and obviously interact with it in a way that causes separation. So we need to start by discovering how a gas can interact with a liquid.
Several household examples illustrate the interaction between a gas and a liquid. For example, put some cold tap water in a saucepan and heat it gently on the stove. We have all done this and seen the result. As the water warms, thousands of tiny bubbles appear and cling to the side of the pan, as illustrated in Figure 2.1. This happens long before the water boils. The bubbles are obviously gas − but what gas is it, and where did it come from?
Gases dissolve less at higher temperature. Heating the water expels the dissolved gas, visible as a cluster of small bubbles. 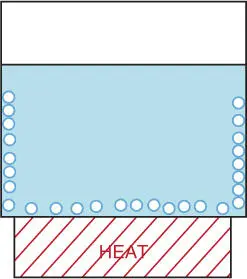
Figure 2.1Gases Dissolve in Liquids.
A moment's thought should reveal the answer. The gas can't be steam because steam bubbles can't exist under water unless the water is boiling. The gas can't be hydrogen because water doesn't decompose at this low temperature. No, the gas must be coming from something dissolved in the water, most likely oxygen and nitrogen from the air.
This first example demonstrates two important principles:
Gases dissolve in liquids.
Gases dissolve less in hot liquids than they do in cold liquids.
Another common example is the bottle of champagne illustrated in Figure 2.2. Closely inspecting the unopened bottle, we see very little gas; the bottle is nearly full of liquid. Yet, upon popping the cork, an enormous quantity of gas suddenly appears. This gas is carbon dioxide, a different gas than in our first example. The liquid is not quite the same either, but it's mainly water. And we can be sure that pure soda water would behave in exactly the same way.
Gases dissolve less at lower pressure. Popping the cork lets the pressure drop, releasing lots of bubbles. 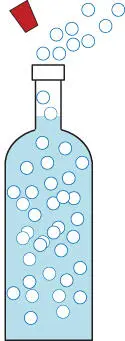
Figure 2.2A Different Gas.
Again, where did the gas come from? There is only one place that it could have been hiding; it was dissolved in the liquid. This confirms our theory that gases dissolve in liquids, but far more was dissolved this time; the solubilityof carbon dioxide in water is greater than the solubility of air in water.
This second example demonstrates two additional principles:
Gases dissolve less at low pressure than they do at high pressure.
Some gases dissolve in a given liquid more than other gases do.
A final example (not illustrated) is what happens when you heat cooking oil in a pan. No bubbles appear. Apparently, the oil doesn't dissolve any air − and it won't dissolve much carbon dioxide either!
This last example gives a fifth principle:
Some liquids dissolve more of a gas than other liquids do.
These five principles are all the science you will need to truly understand what happens in a column. They remind you that a gas can dissolve in a liquid and that the amount of gas that can dissolve depends on only four simple variables; the temperature and pressure, the type of gas, and the type of liquid.
That's it. Nothing else affects the solubility of a gas in a liquid.
The four variables are very easy to understand, yet they are the hidden foundation of all gas chromatography. Let's see how that can be …
In most process gas chromatographs, three of the four variables are closely controlled and do not vary:
The column temperature is held constant.
The carrier gas pressure is held constant.
The liquid phase is predetermined and doesn't change.
The fourth and most important variable is due to the different gases in the sample. And this is the real cause of chromatographic separation:
Different gases have different solubility in the liquid phase.
Читать дальше














