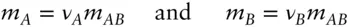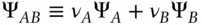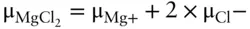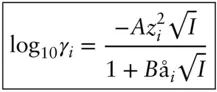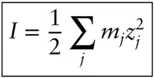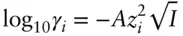The concentration of a salt consisting of ν Amoles of cation A and ν Bmoles of cation B is related to the concentration of its constituent ionic species as:
(3.72) 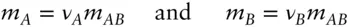
By convention, the thermodynamic properties of ionic species A and B are related to those of the salt AB by:
(3.73) 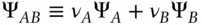
where Ψ is some thermodynamic property. Thus the chemical potential of MgCl 2is related to that of Mg 2+and Cl –as:
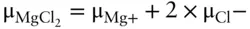
The same holds for enthalpy of formation, entropy, molar volume, and so on.
A final important convention is that the partial molar properties and energies of formation for the proton (H + ) are taken to be zero under all conditions .
3.7.3 Activities in electrolytes
The assumption we made for ideal solution behavior was that interactions between molecules (species might be a better term in the case of electrolyte solutions) of solute and molecules of solvent were not different from those interactions between solvent ions only. In light of the discussion of aqueous solutions earlier, we can see this is clearly not going to be the case for an electrolyte solution. We have seen significant deviations from ideality even where the components have no net charge (e.g., water–ethanol); we can expect greater deviations due to electrostatic interactions between charged species.
The nature of these interactions suggests that a purely macroscopic viewpoint, which takes no account of molecular and ionic interactions, may have severe limitations in predicting equilibria involving electrolyte solutions. Thus, chemists and geochemists concerned with the behavior of electrolytes have had to incorporate a microscopic viewpoint into electrolyte theory. On the other hand, they did not want to abandon entirely the useful description of equilibria based on thermodynamics. We have already introduced concepts, the activity and the activity coefficient, which allow us to treat nonideal behavior within a thermodynamic framework. The additional task imposed by electrolyte solutions, and indeed all real solutions, therefore, is not to rebuild the framework, but simply to determine activities from readily measurable properties of the solution . The dependence of all partial molar properties of a solute on concentration can be determined once the activity coefficient and its temperature and pressure dependence are known.
3.7.3.1 The Debye–Hückel and Davies equations
Both solvent–solute and solute–solute interactions in electrolytes give rise to excess free energies and nonideal behavior. By developing a model to account for these two kinds of interactions, we can develop an equation that will predict the activity of ions in electrolyte solution.
In an electrolyte solution, each ion will exert an electrostatic force on every other ion. These forces will decrease with the increase in square of distance between ions. The forces between ions will be reduced by the presence of water molecules, due to its dielectric nature. As total solute concentration increases, the mean distance between ions will decrease. Thus, we can expect that activity will depend on the total ionic concentration in the solution. The extent of electrostatic interaction will also obviously depend on the charge of the ions involved: the force between Ca 2+and Mg 2+ions will be greater at the same distance than between Na +and K +ions.
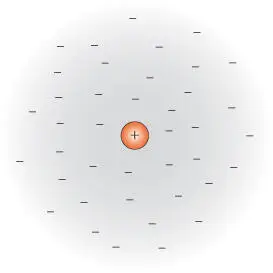
Figure 3.14 An ion surrounded by a cloud of oppositely charged ions, as assumed in Debye–Hückel theory.
In the Debye–Hückel theory ( Figure 3.14), a given ion is considered to be surrounded by an atmosphere or cloud of oppositely charged ions (this atmosphere is distinct from, and unrelated to, the solvation shell). If it were not for the thermal motion of the ions, the structure would be analogous to that of a crystal lattice, though considerably looser. Thermal motion, however, tends to destroy this structure. The density of charge in this ion atmosphere increases with the square root of the ionic concentrations but increases with the square of the charges on those ions. The dielectric effect of intervening water molecules will tend to reduce the interaction between ions. Debye–Hückel theory also assumes the following:
All electrolytes are completely dissociated into ions.
The ions are spherically symmetrical charges (hard spheres).
The solvent is structureless; the sole property is its permittivity.
The thermal energy of ions exceeds the electrostatic interaction energy.
With these assumptions, Debye and Hückel (1923) used the Poisson–Boltzmann equation, which describes the electrostatic interaction energy between an ion and a cloud of opposite charges, to derive the following relationship (see Morel and Hering, 1993, for the full derivation):
(3.74) 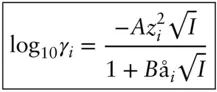
I is ionic strength, in units of molality or molarity, calculated as:
(3.75) 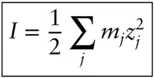
where m is the concentration and z the ionic charge. The parameter å is known as the hydrated ionic radius, or effective radius (significantly larger than the radius of the same ion in a crystal). A and B constants are known as solvent parameters and are functions of T and P . Equation 3.74is known as the Debye–Hückel extended law ; we will refer to it simply as the Debye–Hückel equation . Table 3.2asummarizes the Debye–Hückel solvent parameters over a range of temperatures and Table 3.2bgives values of å for various ions (and see Example 3.3).
For very dilute solutions, the denominator of eqn. 3.74approaches 1 (because I approaches 0), hence eqn. 3.74becomes:
(3.76) 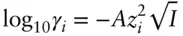
Table 3.2a Debye–Hückel solvent parameters.
| T°C |
A |
B (10 8cm) |
| 0 |
0.4911 |
0.3244 |
| 25 |
0.5092 |
0.3283 |
| 50 |
0.5336 |
0.3325 |
| 75 |
0.5639 |
0.3371 |
| 100 |
0.5998 |
0.3422 |
| 125 |
0.6416 |
0.3476 |
| 150 |
0.6898 |
0.3533 |
| 175 |
0.7454 |
0.3592 |
| 200 |
0.8099 |
0.3655 |
| 225 |
0.8860 |
0.3721 |
| 250 |
0.9785 |
0.3792 |
| 275 |
1.0960 |
0.3871 |
| 300 |
1.2555 |
0.3965 |
From Helgeson and Kirkham (1974).
Читать дальше