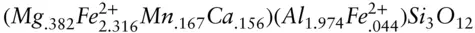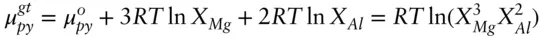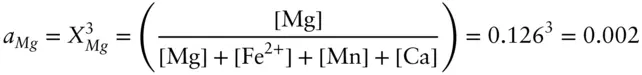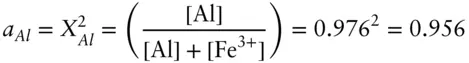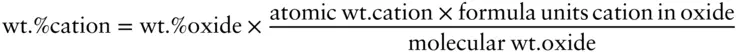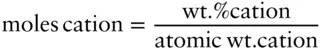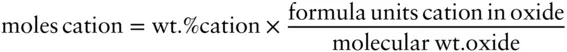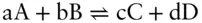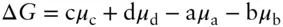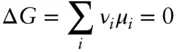A slight complication arises when more than one ion occupies a structural site in the pure phase. For example, suppose we wish to calculate the activity of phlogopite (KMg 3Si 3AlO 10(OH) 2) in a biotite of composition K 0.8Ca 0.2(Mg 0.17Fe 0.83) 3Si 2.8Al 1.2O 10(OH) 2. The tetrahedral site is occupied by Si and Al in the ratio of 3:1 in the pure phase end members. If we were to calculate the activity of phlogopite in pure phlogopite using eqn. 3.80, the activities in the tetrahedral site would contribute only 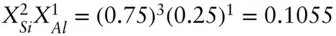 in the pure phase. So we would obtain an activity of 0.1055 instead of 1 for phlogopite in pure phlogopite. Since the activity of a phase component must be one when it is pure, we need to normalize the result. Thus, we apply a correction by multiplying by the raw activity we obtain from 3.92 by 1/(0.1055) = 9.481, and thus obtain an activity of phlogopite of 1.
in the pure phase. So we would obtain an activity of 0.1055 instead of 1 for phlogopite in pure phlogopite. Since the activity of a phase component must be one when it is pure, we need to normalize the result. Thus, we apply a correction by multiplying by the raw activity we obtain from 3.92 by 1/(0.1055) = 9.481, and thus obtain an activity of phlogopite of 1.
Example 3.4Calculating activities using the mixing-on-site model
Sometimes it is desirable to calculate the activities of pure end-member components in solid solutions. Garnet has the general formula X 3Y 2Si 3O 12. Calculate the activity of pyrope, Mg 3Al 2Si 3O 12, in a garnet solid solution of composition:
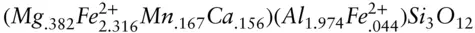
Answer: The chemical potential of pyrope in garnet contains mixing contributions from both Mg in the cubic site and Al in the octahedral site:
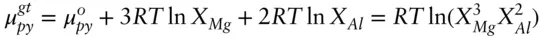
The activity of pyrope is thus given by:

In the example composition above, the activity of Mg is:
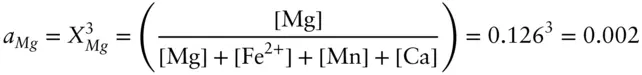
and that of Al is:
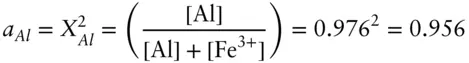
The activity of pyrope in the garnet composition above is 0.002 × 0.956 = 0.00191. There is, of course, no mixing contribution from the tetrahedral site because it is occupied only by Si in both the solution and the pure pyrope phase.
3.8.2 Local charge balance model
Yet another model for the calculation of activities in ideal solid solutions is the local charge balance model. A common example is the substitution of Ca for Na in the plagioclase solid solution (NaAlSi 3O 8–CaAl 2Si 2O 8). To maintain charge balance, the substitution of Ca 2+for Na +in the octahedral site requires substitution of Al 3+for Si 4+in the tetrahedral site. In this model, the activity of the end-member of phase component is equal to the mole fraction of the component (see Example 3.5).
Example 3.5Activities using the local charge balance model
Given the adjacent analysis of a plagioclase crystal, what are the activities of albite and anorthite in the solution?
Plagioclase Analysis
| Oxide |
Wt. percent |
| SiO 2 |
44.35 |
| Al 2O 3 |
34.85 |
| CaO |
18.63 |
| Na 2O |
0.79 |
| K 2O |
0.05 |
Answer : According to the local charge balance model , the activity of albite will be equal to the mole fraction of Na in the octahedral site. To calculate this, we first must convert the weight percent oxides to formula units of cation. The first step is to calculate the moles of cation from the oxide weight percentages. First, we can convert weight percent oxide to weight percent cation using the formula:
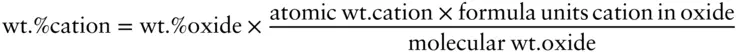
Next, we calculate the moles of cation:
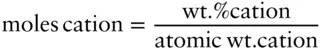
Combining these two equations, the atomic wt. cation terms cancel and we have:
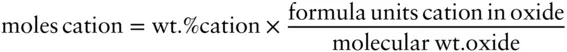
Next, we want to calculate the number of moles of each cation per formula unit. A general formula for feldspar is: XY 4O 8, where X is Na, K, or Ca in the A site and Y is Al or Si in the tetrahedral site. So to calculate formula units in the A site, we divide the number of moles of Na, K, and Ca by the sum of moles of Na, K, and Ca. To calculate formula units in the tetrahedral site, we divide the number of moles of Al and Si by the sum of moles of Al and Si and multiply by 4, since there are four ions in this site. Since the number of oxygens is constant, we can refer to these quantities as the moles per eight oxygens. The following table shows the results of these calculations.
Cation formula units
|
Mol. wt. oxide |
Moles cation |
Moles per 8 oxygens |
| Si |
60.06 |
0.7385 |
2.077 |
| Al |
101.96 |
0.6836 |
1.923 |
| Ca |
56.08 |
0.3322 |
0.926 |
| Na |
61.98 |
0.0255 |
0.071 |
| K |
94.2 |
0.0011 |
0.003 |
The activity of albite is equal to the mole fraction of Na, 0.07; the activity of anorthite is 0.93.
3.9 EQUILIBRIUM CONSTANTS
Now that we have introduced the concepts of activity and activity coefficients, we are ready for one of the most useful parameters in physical chemistry: the equilibrium constant. Though we can predict the equilibrium state of a system, and therefore the final result of a chemical reaction, from the Gibbs free energy alone, the equilibrium constant is a convenient and succinct way express this. As we shall see, it is closely related to, and readily derived from, the Gibbs free energy.
3.9.1 Derivation and definition
Consider a chemical reaction such as:
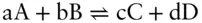
carried out under isobaric and isothermal conditions. The Gibbs free energy change of this reaction can be expressed as:
(3.81) 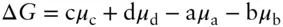
At equilibrium, ΔG must be zero. A general expression then is:
(3.82) 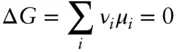
Читать дальше

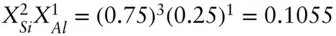 in the pure phase. So we would obtain an activity of 0.1055 instead of 1 for phlogopite in pure phlogopite. Since the activity of a phase component must be one when it is pure, we need to normalize the result. Thus, we apply a correction by multiplying by the raw activity we obtain from 3.92 by 1/(0.1055) = 9.481, and thus obtain an activity of phlogopite of 1.
in the pure phase. So we would obtain an activity of 0.1055 instead of 1 for phlogopite in pure phlogopite. Since the activity of a phase component must be one when it is pure, we need to normalize the result. Thus, we apply a correction by multiplying by the raw activity we obtain from 3.92 by 1/(0.1055) = 9.481, and thus obtain an activity of phlogopite of 1.