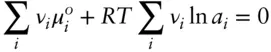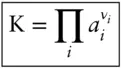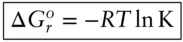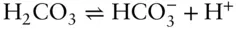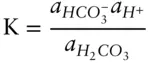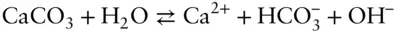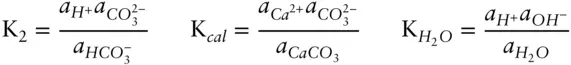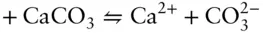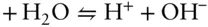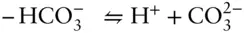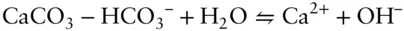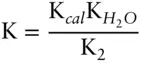where ν iis the stoichiometric coefficient of species i . Equilibrium in such situations need not mean that all the reactants (i.e., those phases on the left side of the equation) are consumed to leave only products. Indeed, this is generally not so. Substituting eqn. 3.46into 3.82 we obtain:
(3.83) 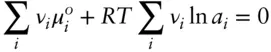
or:
(3.84) 
The first term is simply the standard state Gibbs free energy change, ΔG° , for the reaction. There can be only one fixed value of ΔG° for a fixed standard state pressure and temperature, and therefore of the activity products. The activity products are therefore called the equilibrium constant K, familiar from elementary chemistry:
(3.85) 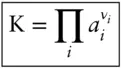
Substituting eqn. 3.85into 3.84and rearranging, we see that the equilibrium constant is related to the Gibbs free energy change of the reaction by the equation:
(3.86) 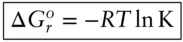
At this point, it is worth saying some more about standard states . We mentioned that one is free to choose a standard state, but there are pitfalls. In general, there are two kinds of standard states, fixed pressure–temperature standard states and variable P – T standard states. If you chose a fixed temperature standard state, then eqn. 3.86is only valid at that standard-state temperature. If you chose a variable-temperature standard state, then eqn. 3.86is valid for all temperatures, but ΔG° is then a function of temperature. The same goes for pressure. Whereas most thermodynamic quantities we have dealt with thus far are additive, equilibrium constants are multiplicative (see Example 3.6).
Let's attempt to understand the implications of eqn. 3.85. Consider the dissociation of carbonic acid, an important geologic reaction:
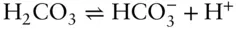
For this particular case, eqn. 3.85is expressed as:
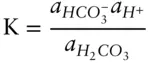
The right side of the equation is a quotient, the product of the activities of the products divided by the product of the activities of the reactants and is called the reaction quotient . At equilibrium, the reaction quotient is equal to the equilibrium constant. The equilibrium constant therefore allows us to predict the relative amounts of products and reactants that will be present when a system reaches equilibrium.
Suppose now that we prepare a beaker of carbonic acid solution; it is not hard to prepare: we just allow pure water to equilibrate with the atmosphere. Let's simplify things by assuming that this is an ideal solution. This allows us to replace activities with concentrations (the concentration units will dictate how we define the equilibrium constant; see below). When the solution has reached equilibrium, just enough carbonic acid will have dissociated so that the reaction quotient will be equal to the equilibrium constant. Now let's add some H +ions, perhaps by adding a little HCl. The value of the reaction quotient increases above that of the equilibrium constant and the system is no longer in equilibrium. Systems will always respond to disturbances by moving toward equilibrium (how fast they respond is another matter, and one that we will address in Chapter 5). The system will respond by adjusting the concentrations of the three species until equilibrium is again achieved; in this case, hydrogen and bicarbonate ions will combine to form carbonic acid until the reaction quotient again equals the equilibrium constant. We can also see that had we reduced the number of hydrogen ions in the solution (perhaps by adding a base), the reaction would have been driven the other way (i.e., hydrogen ions would be produced by dissociation). Equation 3.85is known as the law of mass action , which we can state more generally: Changing the concentration of one species in a system at equilibrium will cause a reaction in a direction that minimizes that change .
Example 3.6Manipulating reactions and equilibrium constant expressions
Often we encounter a reaction for which we have no value of the equilibrium constant. In many cases, however, we can derive an equilibrium constant by considering the reaction of interest to be the algebraic sum of several reactions for which we do have equilibrium constant values. For example, the concentration of carbonate ion is often much lower than that of the bicarbonate ion. In such cases, it is more convenient to write the reaction for the dissolution of calcite as:
(3.87) 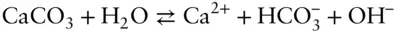
Given the following equilibrium constants, what is the equilibrium constant expression for the above reaction?
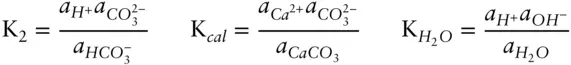
Answer: Reaction 3.87can be written as the algebraic sum of three reactions:
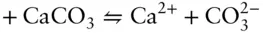
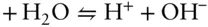
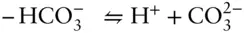
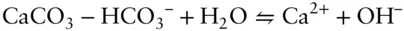
The initial inclination might be to think that if we can sum the reactions, the equilibrium constant of the resulting reaction is the sum of the equilibrium constants of the components. However, this is not the case. Whereas we sum the reactions, we take the product of the equilibrium constants. Thus, our new equilibrium constant is:
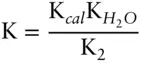
For several reasons (chief among them that equilibrium constants can be very large or very small numbers), it is often more convenient to work with the log of the equilibrium constant. A commonly used notation is pK. pK is the negative logarithm (base 10) of the corresponding equilibrium constant (note this notation is analogous to that used for pH). The pK's sum and our equilibrium constant expression is:
Читать дальше