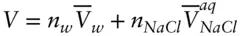A final interesting property of water is that some fraction of water molecules will autodissociate . In pure water at standard state conditions, one in every 10 −7molecules will dissociate to form H +and OH –ions. Although in most thermodynamic treatments the protons produced in this process are assumed to be free ions, most will combine with water molecules to form H 3O +ions. OH −is called the hydroxyl ion; the H 3O +is called hydronium .
3.7.2 Some definitions and conventions
The first two terms we need to define are solvent and solute. Solvent is the substance present in greatest abundance in a solution; in the electrolyte solutions that we will discuss here, water is always the solvent. Solute refers to the remaining substances present in solution. Thus, in seawater, water is the solvent and NaCl, CaSO 4, and so on, are the solutes. We may also refer to the individual ions as solutes.
3.7.2.1 Concentration units
Geochemists concerned with aqueous solutions commonly use a variety of concentration units other than mole fraction. The first is molality (abbreviated as lower-case m ), which is moles of solute per kg of solvent (H 2O). Molality can be converted to moles solute per moles solvent units by dividing by 55.51 mol/kg. A second unit is molarity (abbreviated as uppercase M ), which is moles of solute per liter of solution. To convert molality to mole fraction, we would divide by the molecular weight of solvent and use the rational activity coefficient. Natural solutions are often sufficiently dilute that the difference between molality and molarity is trivial (seawater, a relatively concentrated natural solution, contains only 3.5 weight percent dissolved solids). Another common unit is weight fraction (i.e., grams per gram solution), which may take several forms, such as weight percentage, parts per thousand, or parts per million (abbreviated %, ppt or ‰, ppm or mg/kg). To convert to mole fraction, one simply divides the weight of solute and H 2O by the respective molecular weights.
One of the most common parameters in aqueous geochemistry is pH . pH is defined as the negative logarithm of the hydrogen ion activity:
(3.68) 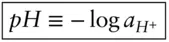
3.7.2.3 Standard state and other conventions
The first problem we must face in determining activities in electrolyte solutions is specifying the standard state. With gases, the standard state is generally the pure substance (generally at 298 K and 1 atm), but this is generally not a reasonable choice for electrolytes. A NaCl solution will become saturated at about 0.1 X NaCl, and crystalline NaCl has very different properties from NaCl in aqueous solution. By convention, a hypothetical standard state of unit activity at 1 molal concentration is chosen:
(3.69) 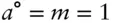
Activity is generally given units of molality in this case (it is dimensionless, as we defined it in eqn. 3.45), so that in this hypothetical standard state, activity equals molality. The standard state is hypothetical because, for most electrolytes, the activity will be less than 1 in a 1 m (molal) solution. Because the standard state generally is unattainable in reality, we must also define an attainable reference state , from which experimental measurements can be extrapolated. By convention, the reference state is that of an infinitely dilute solution – the Henry's law state. For multicomponent solutions, we also specify that the concentrations of all other components be held constant. Hence the reference state is:
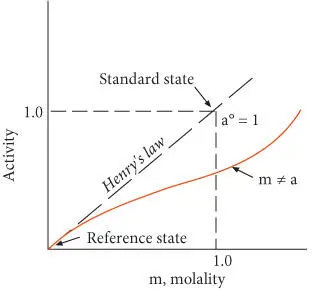
Figure 3.11 Relationship of activity and molality, reference state, and standard state for aqueous solutions. After Nordstrom and Munoz (1986).
(3.70) 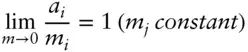
This convention is illustrated in Figure 3.11. In such solutions, the activity coefficient can be shown to depend on the charge of the ion, its concentration, and the concentration of other ions in the solution as well as temperature and other parameters of the solute. Comparing eqn. 3.70with 3.46 and 3.48, we see that under these conditions, the activity coefficient is 1. By referring to infinite dilution, we are removing the effect of solute–solute interactions. The standard state properties of an electrolyte solution therefore only take account of solvent–solute interactions.
Clearly, it is impossible to measure the properties of the solute, such as chemical potential or molar volume, at infinite dilution. In practice, this problem is overcome by measuring properties at some finite dilution and extrapolating the result to infinite dilution. Indeed, even at finite concentrations, it is not possible to measure directly many properties of electrolytes. Volume is a good example. One cannot measure the volume of the solute, but one can measure the volume change of the solution as a function of concentration of the solute. Then by assuming that the partial molar volume of water does not change, a partial molar volume of the solute can be calculated. This is called the apparent molar volume , 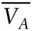 . The apparent molar volume of NaCl as a function of molarity is shown in Figure 3.12. In essence, this convention assigns all deviations from nonideality to the solute and allows us to use the partial molar volume of pure water in the place of the true, but unknown, molar volume of water in the solution. Thus the volume of NaCl solution is given by:
. The apparent molar volume of NaCl as a function of molarity is shown in Figure 3.12. In essence, this convention assigns all deviations from nonideality to the solute and allows us to use the partial molar volume of pure water in the place of the true, but unknown, molar volume of water in the solution. Thus the volume of NaCl solution is given by:
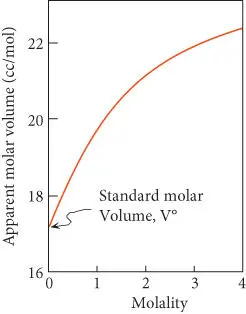
Figure 3.12 Apparent molar volume of NaCl in aqueous solution as a function of molality. The standard molar volume, V°, is the apparent molar volume at infinite dilution.
(3.71) 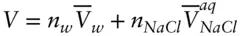
This convention leads to some interesting effects. For example, the apparent molar volume of magnesium sulfate increases with pressure, and many other salts, including NaCl ( Figure 3.13), exhibit the same behavior. Just as curiously, the apparent molar volume of sodium chloride in saturated aqueous solution becomes negative above ∼200°C ( Figure 3.13). Many other salts show the same effect. These examples emphasize the “apparent” nature of molar volume when defined in this way. Of course, the molar volume of NaCl does not actually become negative; rather, this is the result of the interaction between Na +and Cl –and H 2O (electrostriction) and the convention of assigning all nonideality to sodium chloride.
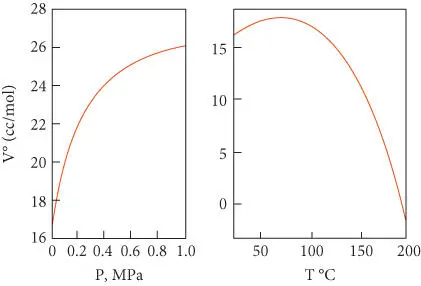
Figure 3.13 Standard molar volume of NaCl in aqueous solution as a function of temperature and pressure. Based on the data of Helgeson and Kirkham (1976).
Читать дальше

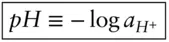
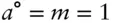

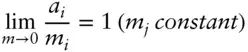
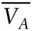 . The apparent molar volume of NaCl as a function of molarity is shown in Figure 3.12. In essence, this convention assigns all deviations from nonideality to the solute and allows us to use the partial molar volume of pure water in the place of the true, but unknown, molar volume of water in the solution. Thus the volume of NaCl solution is given by:
. The apparent molar volume of NaCl as a function of molarity is shown in Figure 3.12. In essence, this convention assigns all deviations from nonideality to the solute and allows us to use the partial molar volume of pure water in the place of the true, but unknown, molar volume of water in the solution. Thus the volume of NaCl solution is given by: