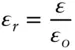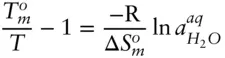
For a reasonably dilute solution, the activity of water will approximately equal its mole fraction, so that:
(3.66) 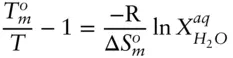
The entropy of melting is always positive, and since X is always less than 1, the left-hand side of eqn. 3.66must always be positive. Thus, the ratio 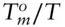 must always be greater than 1. So the temperature at which an aqueous solution will freeze will always be less than the melting point of pure water. Salting of roads is not a question of geochemical interest, but there are many examples of depression of the freezing point of geological interest. For example, the freezing point of the ocean is about –2°C, and this phenomenon is important in igneous petrology, as we shall see in the next chapter. A related phenomenon of geological interest is elevation of the boiling point of a liquid: for example, hydrothermal solutions boil at temperatures significantly above that of pure water. Can you demonstrate that elevation of the boiling point of an ideal solution depends only on the mole fraction of the solute?
must always be greater than 1. So the temperature at which an aqueous solution will freeze will always be less than the melting point of pure water. Salting of roads is not a question of geochemical interest, but there are many examples of depression of the freezing point of geological interest. For example, the freezing point of the ocean is about –2°C, and this phenomenon is important in igneous petrology, as we shall see in the next chapter. A related phenomenon of geological interest is elevation of the boiling point of a liquid: for example, hydrothermal solutions boil at temperatures significantly above that of pure water. Can you demonstrate that elevation of the boiling point of an ideal solution depends only on the mole fraction of the solute?
3.7 ELECTROLYTE SOLUTIONS
Electrolyte solutions are solutions in which the solute dissociates to form ions, which facilitate electric conduction. Seawater is an obvious example of a natural electrolyte solution, but all natural waters are also electrolytes, though generally more dilute ones. These solutions, which Lavoisier *called the “rinsings of the Earth,” are of enormous importance in many geologic processes.
3.7.1 The nature of water and water–electrolyte interaction
There is perhaps no compound more familiar to us than H 2O. Commonplace though it might be, H 2O is the most remarkable compound in nature. Its unusual properties include: the highest heat capacity of all solids and liquids except ammonia, the highest latent heat of vaporization of all substances, the highest surface tension of all liquids, its maximum density is at 4°C, with density decreasing below that temperature (negative coefficient of thermal expansion), the solid form is less dense than the liquid (negative Clapeyron slope), and finally, it is the best solvent known, dissolving more substances and in greater quantity than any other liquid. We will digress here briefly to consider the structure and properties of H 2O and the nature of water–electrolyte interactions from a microscopic perspective.
Many of the unusual properties of water arise from its nonlinear polar structure, which is illustrated in Figure 3.9a. The polar nature of water gives rise to van der Waals forces and the hydrogen bond discussed in Chapter 1. The hydrogen bond, which forms between hydrogen and oxygen atoms of adjacent molecules, imposes a dynamic partial structure on liquid water ( Figure 3.9b). These bonds continually break and new ones reform, and there is always some fraction of unassociated molecules. On average, each water molecule is coordinated by four other water molecules. When water boils, all hydrogen bonds are broken. The energy involved in breaking these bonds accounts for the high heat of vaporization.
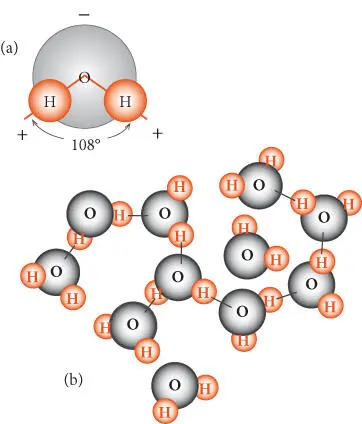
Figure 3.9 (a) Structure of the water molecule. Bond angle in the liquid phase is 108°, and 105° in the gas. The hydrogens retain a partial positive charge and the oxygen retains a partial positive charge. (b) Partial structure present in liquid water. Lines connecting adjacent molecules illustrate hydrogen bonds.
The dissolving power of water is due to its dielectric nature. A dielectric substance is one that reduces the forces acting between electric charges. When placed between two electrically charged plates (a capacitor), water molecules will align themselves in the direction of the electric field. As a result, the molecules oppose the charge on the plates and effectively reduce the transmission of the electric field. The permittivity , ε, of a substance is the measure of this effect. The relative permittivit y, or dielectric constant , ε r, of a substance is defined as the ratio of the capacitance observed when the substance is placed between the plates of a capacitor to the capacitance of the same capacitor when a vacuum is present between the plates:
(3.67) 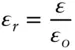
where ε 0is the permittivity of a vacuum (8.85 × 10 −12C 2/J m). The relative permittivity of water is 78.54 at 25°C and 1 atm. For comparison, the relative permittivity of methane, a typical nonpolar molecule, is 1.7.
Water molecules surrounding a dissolved ion will tend to align themselves to oppose the charge of the ion. This insulates the ion from the electric field of other ions. This property of water accounts in large measure for its dissolving power. For example, we could easily calculate that the energy required to dissociate NaCl (i.e., the energy required to move Na +and Cl –ions from their normal interatomic distance in a lattice, 236 pm, to infinite separation) is about 585 kJ/mol. Because water has a dielectric constant of about 80, this energy is reduced by a factor of 80, so only 7.45 kJ are required for dissociation.
The charged nature of ions and the polar nature of water result in the solvation of dissolved ions. Immediately adjacent to the ion, water molecules align themselves to oppose the charge on the ion, such that the oxygen of the water molecule will be closest to a cation ( Figure 3.10). These water molecules are called the first solvation shell or layer and they are effectively bound to the ion, moving with it as it moves. Beyond the first solvation shell is a region of more loosely bound molecules that are only partially oriented, called the second solvation shell or layer. The boundary of this latter shell is diffuse: there is no sharp transition between oriented and unaffected water molecules. The energy liberated in this process, called the solvation energy , is considerable. For NaCl, for example, it is −765kJ/mol (it is not possible to deduce the solvation energies of Na +and Cl –independently). The total number of water molecules bound to the ion is called the solvation number . Solvation effectively increases the electrostatic radius of cations by about 90 pm and of anions by about 10 pm per unit of charge.
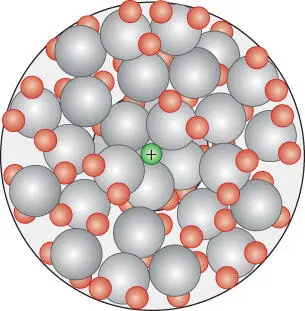
Figure 3.10 Solvation of a cation in aqueous solution. In the first solvation shell, water molecules are bound to the cation and oriented so that the partial negative charge on the oxygen faces the cation. In the second solvation shell, molecules are only loosely bound and partially oriented.
An additional effect of solvation is electrostriction . Water molecules in the first solvation sphere are packed more tightly than they would otherwise be. This is true, to a lesser extent, of molecules in the secondary shell. In addition, removal of molecules from the liquid water structure causes partial collapse of this structure. The net effect is that the volume occupied by water in an electrolyte solution is less than in pure water, which can lead to negative apparent molar volumes of solutes, as we shall see. The extent of electrostriction depends strongly on temperature and pressure.
Читать дальше

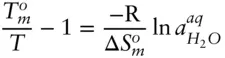
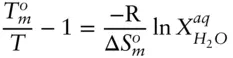
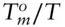 must always be greater than 1. So the temperature at which an aqueous solution will freeze will always be less than the melting point of pure water. Salting of roads is not a question of geochemical interest, but there are many examples of depression of the freezing point of geological interest. For example, the freezing point of the ocean is about –2°C, and this phenomenon is important in igneous petrology, as we shall see in the next chapter. A related phenomenon of geological interest is elevation of the boiling point of a liquid: for example, hydrothermal solutions boil at temperatures significantly above that of pure water. Can you demonstrate that elevation of the boiling point of an ideal solution depends only on the mole fraction of the solute?
must always be greater than 1. So the temperature at which an aqueous solution will freeze will always be less than the melting point of pure water. Salting of roads is not a question of geochemical interest, but there are many examples of depression of the freezing point of geological interest. For example, the freezing point of the ocean is about –2°C, and this phenomenon is important in igneous petrology, as we shall see in the next chapter. A related phenomenon of geological interest is elevation of the boiling point of a liquid: for example, hydrothermal solutions boil at temperatures significantly above that of pure water. Can you demonstrate that elevation of the boiling point of an ideal solution depends only on the mole fraction of the solute?