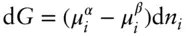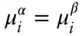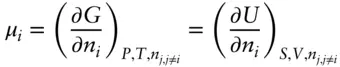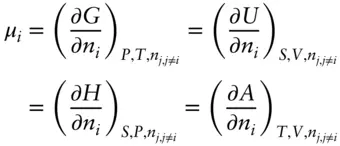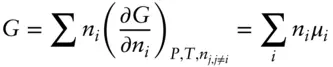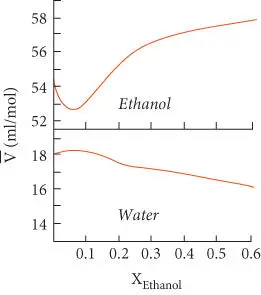
Figure 3.5 Variation of the partial molar volumes of water and ethanol as a function of the mole fraction of ethanol in a binary solution. This figure also illustrates the behavior of a very nonideal solution. After Nordstrom and Munoz (1986).
The second expression in eqn. 3.11says that the volume of a phase is the sum of the partial molar volumes of the components times the number of moles of each component present. Thus, the volume of plagioclase would be the sum of the partial molar volumes of the albite and anorthite components weighted by the number of moles of each.
Another example might be a solution of water and ethanol. The variation of the partial molar volumes of water and ethanol in a binary solution is illustrated in Figure 3.5. This system illustrates very clearly why the qualification “for an infinitesimal addition” is always added: the value of a partial molar quantity of a component may vary with the amount of that component present.
Equation 3.11can be generalized to all partial molar quantities and also expresses an important property of partial molar quantities: an extensive variable of a system or phase is the sum of its partial molar quantities for each component in the system . In our example above, this means that the volume of plagioclase is the sum of the partial molar volume of the albite and anorthite components.
Generally, we find it more convenient to convert extensive properties to intensive properties by dividing by the total number of moles in the system, Σ n . Dividing both sides of eqn. 3.11by Σ n we have:
(3.12) 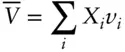
This equation says that the molar volume of a substance is the sum of the partial molar volumes of its components times their mole fractions. For a pure phase, the partial molar volume equals the molar volume since X = 1 .
3.4.2 Definition of chemical potential and relationship to Gibbs free energy
We define μ as the chemical potential , which is simply the partial molar Gibbs free energy:
(3.13) 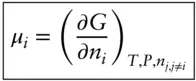
The chemical potential thus tells us how the Gibbs free energy will vary with the number of moles, n i, of component i holding temperature, pressure, and the number of moles of all other components constant. We said that the Gibbs free energy of a system is a measure of the capacity of the system to do chemical work. Thus, the chemical potential of component i is the amount by which this capacity to do chemical work is changed for an infinitesimal addition of component i at constant temperature and pressure. In a NiCd battery (common rechargeable batteries), for example, the chemical potential of Ni in the battery (our system) is a measure of the capacity of the battery to provide electrical energy per mole of additional Ni for an infinitesimal addition.
The total Gibbs free energy of a system will depend on composition as well as on temperature and pressure. The equations we introduced for Gibbs free energy in Chapter 2fully describe the Gibbs free energy only for single component systems or systems containing only pure phases. The Gibbs free energy change of a phase of variable composition is fully expressed as:
(3.14) 
3.4.3 Properties of the chemical potential
We now want to consider two important properties of the chemical potential. To illustrate these properties, consider a simple two-phase system in which an infinitesimal amount of component i is transferred from phase β to phase α , under conditions where T , P , and the amount of other components is held constant in each phase. One example of such a reaction would be the transfer of Pb from a hydrothermal solution to a sulfide mineral phase. The chemical potential expresses the change in Gibbs free energy under these conditions:
(3.15) 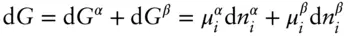
since we are holding everything else constant, atoms gained by α must be lost by β , so 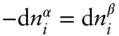 and:
and:
(3.16) 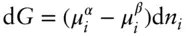
At equilibrium, d G = 0, and therefore
(3.17) 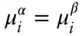
Equation 3.17reflects a very general and very important relationship, namely:
In a system at equilibrium, the chemical potential of every component in a phase is equal to the chemical potential of that component in every other phase in which that component is present.
Equilibrium is the state toward which systems will naturally transform. The Gibbs free energy is the chemical energy available to fuel these transformations. We can regard differences in chemical potentials as the forces driving transfer of components between phases . In this sense, the chemical potential is similar to other forms of potential energy, such as gravitational or electromagnetic. Physical systems spontaneously transform so as to minimize potential energy. Thus, for example, water on the surface of the Earth will move to a point where its gravitational potential energy is minimized – downhill. Just as gravitational potential energy drives this motion, the chemical potential drives chemical reactions, and just as water will come to rest when gravitational energy is minimized, chemical reactions will cease when chemical potential is minimized. So in our example above, the spontaneous transfer of Pb between a hydrothermal solution and a sulfide phase will occur until the chemical potentials of Pb in the solution and in the sulfide are equal. At this point, there is no further energy available to drive the transfer.
We defined the chemical potential in terms of the Gibbs free energy. However, in his original work, Gibbs based the chemical potential on the internal energy of the system. As it turns out, however, the quantities are the same:
(3.18) 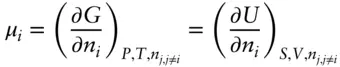
It can be further shown (but we won't) that:
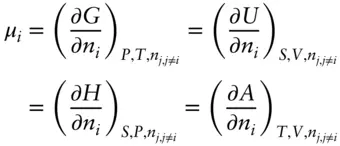
3.4.4 The Gibbs–Duhem relation
Since μ is the partial molar Gibbs free passim energy, the Gibbs free energy of a system is the sum of the chemical potentials of each component:
(3.19) 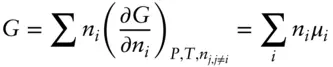
Читать дальше


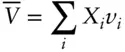
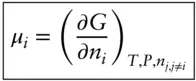

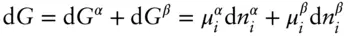
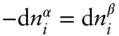 and:
and: