9 *Either a maximum or minimum can occur where the derivative is 0, and a function may have several of both; so some foreknowledge of the properties of the function of interest is useful in using this property.
10 †This equation, which relates microscopic and macroscopic variables, is inscribed on the tombstone of Ludwig Boltzmann (1844–1906), the Austrian physicist responsible for it.
11 †R. Clausius recognized the possibility that molecules might have these three kinds of motion in 1855.
12 *We now understand and interpret this law in terms of quantum physics, but Boltzmann formulated it 30 years before Planck and Einstein laid the foundations of quantum theory. Ludwig Boltzmann's work in the second half of the nineteenth century laid the foundations of statistical mechanics and paved the way for quantum theory in the next century. His work was heavily attacked by other physicists of the time, who felt physics should deal only with macroscopic observable quantities and not with atoms, which were then purely hypothetical constructs. These attacks contributed to increasingly frequent bouts of depression, which ultimately led to Boltzmann's suicide in 1906. Ironically and sadly, this was about the time that Perrin's experiments with Brownian motion, Millikan's oil drop experiment, and Einstein's work on the photoelectric effect confirmed the discrete nature of mass, charge, and energy, and thereby the enduring value of Boltzmann's work.
13 *Albert Einstein (1879–1955), though best known for his relativity theories, was also the founder, along with Max Planck, of quantum physics. His work on the quantum basis of heat capacity of solids was published in 1907. Einstein was born in Ulm, Germany, and published some of his most significant papers while working as a patent clerk in Bern, Switzerland. He later joined the Prussian Academy of Sciences in Berlin. A dedicated and active pacifist, Einstein left Germany when Hitler came to power in 1933. He later joined the Center for Advanced Studies in Princeton, New Jersey.
14 †Peter Debye (1884–1966) was born in Maastricht, Netherlands (as Petrus Debije), but spent much of his early career in Germany, eventually becoming director of the Kaiser-Wilhelm-Institut in Berlin. While he was visiting Cornell University in 1940, Germany invaded Holland and Debye simply remained at Cornell, eventually becoming chairman of the Chemistry Department. Debye made numerous contributions to physics and physical chemistry; we shall encounter his work again in the next chapter.
15 *The Maxwell relations are named for Scottish physicist James Clerk Maxwell (1831–1879), perhaps the most important figure in nineteenth-century physics. He is best known for his work on electromagnetic radiation, but he also made very important contributions to statistical mechanics and thermodynamics.
Chapter 3 Solutions and thermodynamics of multicomponent systems
3.1 INTRODUCTION
In the previous chapter, we introduced thermodynamic tools that allow us to predict the equilibrium mineral assemblage under a given set of conditions. For example, having specified temperature, we were able to determine the pressure at which the assemblage anorthite + forsterite is in equilibrium with the assemblage diopside + spinel + enstatite. In that reaction the minerals had unique and invariant compositions. In the Earth, things are not quite so simple: these minerals are present as solid solutions *, with substitutions of Fe 2+for Mg, Na for Ca, and Cr and Fe 3+for Al, among others. Indeed, most natural substances are solutions; that is, their compositions vary. Water, which is certainly the most interesting substance at the surface of the Earth and perhaps the most important, inevitably has a variety of substances dissolved in it. These dissolved substances are often of primary geochemical interest. More to the point, they affect the chemical behavior of water. For example, the freezing temperature of an aqueous NaCl solution is lower than that of pure water. You may have taken advantage of this phenomenon by spreading salt to de-ice sidewalks and roads.
In a similar way, the equilibrium temperature and pressure of the plagioclase + olivine ⇌ clinopyroxene + spinel + orthopyroxene reaction depends on the composition of these minerals. To deal with this compositional dependence, we need to develop some additional thermodynamic tools, which is the objective of this chapter. This may seem burdensome at first: if it were not for the variable composition of substances, we would already know most of the thermodynamics we need. However, as we will see in Chapter 4, we can use this compositional dependence to advantage in reconstructing conditions under which a mineral assemblage or a hydrothermal fluid formed.
A final difficulty is that the valance state of many elements can vary. Iron, for example, may change from its Fe 2+state to Fe 3+when an igneous rock weathers. The two forms of iron have very different chemical properties; for example, Fe 2+is considerably more soluble in water than is Fe 3+. Another example of this kind of reaction is photosynthesis, the process by which CO 2is converted to organic carbon. These kinds of reactions are called oxidation–reduction, or redox reactions. The energy your brain uses to process the information you are now reading comes from oxidation of organic carbon – carbon originally reduced by photosynthesis in plants. To fully specify the state of a system, we must specify its “redox” state. We treat redox reactions in the final section of this chapter.
Though Chapter 4will add a few more tools to our geochemical toolbox, and treat a number of advanced topics in thermodynamics, it is designed to be optional. With completion of this chapter, you will have a sufficient thermodynamic background to deal with a wide range of phenomena in the Earth, and most of the topics in the remainder of this book.
3.2 PHASE EQUILIBRIA
3.2.1 Some definitions
3.2.1.1 Phase
Phases are real substances that are homogeneous, physically distinct, and (in principle) mechanically separable. For example, the phases in a rock are the minerals present. Amorphous substances are also phases, so glass and opal would be phases. The sugar that won't dissolve in your iced tea is a distinct phase from the tea, but the dissolved sugar is not. Phase is not synonymous with compound . Phases need not be chemically distinct: a glass of ice water has two distinct phases: water and ice. Many solid compounds can exist as more than one phase. Nor need they be compositionally unique: plagioclase, clinopyroxene, olivine, and so on, are all phases even though their composition can vary. A fossil in which the aragonite (CaCO 3) is partially retrograded into calcite (also CaCO 3) consists of two phases, which, although they might be chemically identical, have different crystal structures and hence different properties. Systems and reactions occurring within them that consist of a single phase are referred to as homogeneous ; those systems consisting of multiple phases, and the reactions occurring within them, are referred to as heterogeneous .
Species is somewhat more difficult to define than either phase or component . A species is a chemical entity, generally an element or compound (which may or may not be ionized). The term is most useful in the context of gases and liquids. A single liquid phase, such as an aqueous solution, may contain a number of species. For example, H 2O, H 2CO 3, 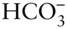 ,
, 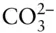 , H +, and OH –are all species commonly present in natural waters. The term species is generally reserved for an entity that actually exists, such as a molecule, ion, or solid, at least on a microscopic scale. This is not necessarily the case with components, as we shall see. The term species is less useful for solids, although it is sometimes applied to the pure end-members of solid solutions and to pure minerals.
, H +, and OH –are all species commonly present in natural waters. The term species is generally reserved for an entity that actually exists, such as a molecule, ion, or solid, at least on a microscopic scale. This is not necessarily the case with components, as we shall see. The term species is less useful for solids, although it is sometimes applied to the pure end-members of solid solutions and to pure minerals.
Читать дальше

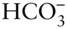 ,
, 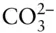 , H +, and OH –are all species commonly present in natural waters. The term species is generally reserved for an entity that actually exists, such as a molecule, ion, or solid, at least on a microscopic scale. This is not necessarily the case with components, as we shall see. The term species is less useful for solids, although it is sometimes applied to the pure end-members of solid solutions and to pure minerals.
, H +, and OH –are all species commonly present in natural waters. The term species is generally reserved for an entity that actually exists, such as a molecule, ion, or solid, at least on a microscopic scale. This is not necessarily the case with components, as we shall see. The term species is less useful for solids, although it is sometimes applied to the pure end-members of solid solutions and to pure minerals.










