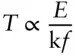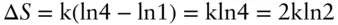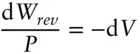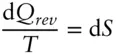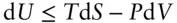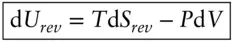(2.55) 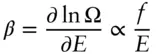
Substituting 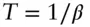 , then
, then
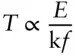
In other words, the temperature of a system is proportional to its energy per degree of freedom.
2.6.2.2 Entropy and volume
Our discussion of entropy might leave the impression that entropy is associated only with heat and temperature. This is certainly not the case. Our first example, that of the gases in the box ( Figure 2.5), is a good demonstration of how entropy changes can also accompany isothermal processes. When the partition is removed and the gases mix, there is an increase in the number of states accessible to the system. Before the partition is removed, there is only one state accessible to the system (here, accessible states means distribution of red and black molecules between the two sides of the box), so Ω = 1. Suppose that after we remove the partition, we find the system state is one where there is one molecule of each kind on each side (the most probable case). There are four such possible configurations, so Ω = 4. The entropy change has thus been
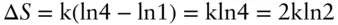
From the macroscopic perspective, we could say that the red gas, initially confined to the left volume, expands into the volume of the entire box, and the black gas expands from the right half to the entire volume. Thus entropy changes accompany volume changes.
It is often said that entropy is a measure of the randomness of a system. From the discussion above, we can understand why. Entropy is a function of the number of states accessible to a system. Because there are more states available to a system when energy or molecules are “evenly” or “randomly” distributed than when we impose a specific constraint on a system (such as the thermal insulation between the blocks or the partition between the gases), there is indeed an association between randomness and entropy. When we remove the insulation between the copper blocks, we allow energy to be randomly distributed between them. In the example of the combustion of gasoline, before combustion all oxygen atoms are constrained to be associated with oxygen molecules. After combustion, oxygen is randomly distributed between water and CO 2molecules.
More precisely, we may say that an increase in entropy of a system corresponds to a decrease in knowledge of it. In the example of our two gases in the box, before the partition is removed, we know all red molecules are located somewhere in the left half of the box and all black ones somewhere in the right half. After the partition is removed, we know only that the molecules are located somewhere within the combined volume. Thus our knowledge of the location of the molecules decreases in proportion to the change in volume. Molecules in ice are located at specific points in the crystal lattice. When ice melts, or evaporates, molecules are no longer constrained to specific locations: there is an increase in entropy of H 2O and a corresponding decrease in our knowledge of molecular positions. When we allowed the two copper blocks to come to thermal equilibrium, entropy increased. There were more possible ways to distribute energy after the blocks equilibrated than before. As a result, we knew less about how energy is distributed after removing the insulation.
As a final point, we emphasize that the second law does not mean we cannot decrease the entropy of a “system.” Otherwise, the organization of molecules we call life would not be possible. However, if the entropy of a system is to decrease, the entropy of its surroundings must increase. Thus, we can use air conditioning to cool a room, but the result is that the surroundings (the “outside”) are warmed by more than the air in the room is cooled. Organisms can grow, but in doing so they inevitably, through consumption and respiration, increase the entropy of their environment. Thus we should not be surprised to find that the entropy of the manure is greater than that of hay plus oxygen.
2.6.3 Integrating factors and exact differentials
A theorem of mathematics states that any inexact differential that is a function of only two variables can be converted to an exact differential. d W is an inexact differential, and d V is an exact differential. Since d W rev= − P d V , d W revcan be converted to a state function by dividing by P since
(2.56) 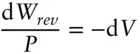
and V is a state function. Variables such as P that convert nonstate functions to state functions are termed integrating factors . Similarly, for a reversible reaction, heat can be converted to the state function entropy by dividing by T :
(2.57) 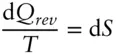
Thus temperature is the integrating factor of heat. Entropy is a state function and therefore an exact differential. Therefore, eqn. 2.57is telling us that although the heat gained or lost in the transformation from state 1 to state 2 will depend on the path taken , for a reversible reaction the ratio of heat gained or lost to temperature will always be the same, regardless of path .
If we return to our example of the combustion of gasoline above, the second law also formalizes our experience that we cannot build a 100% efficient engine: the transformation from state 1 to state 2 cannot be made in such a way that all energy is extracted as work; some heat must be given up as well. In this sense, the second law necessitates the automobile radiator.
Where P – V work is the only work of interest, we can combine the first and second laws as:
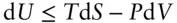
The implication of this equation is that if equilibrium is approached at prescribed S and V , the energy of the system is minimized. For the specific situation of a reversible reaction
where d S = d Q / T , this becomes
(2.58) 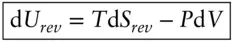
This expresses energy in terms of its natural or characteristic variables , S and V . The characteristic variables of a function are those that give the simplest form of the exact differential. Since neither T nor P may have negative values, we can see from this equation that energy will always increase with increasing entropy (at constant volume) and that energy will decrease with increasing volume (at constant entropy). This equation also relates all the primary state variables of thermodynamics, U, S, T, P , and V . For this reason, it is sometimes called the fundamental equation of thermodynamics . We will introduce several other state variables derived from these five, but these will be simply a convenience.
Читать дальше

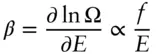
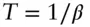 , then
, then