(2.74) 
Thus, the difference between C pand C vwill depend on temperature and pressure for real substances. The terms on the right will always be positive, so that C pwill always be greater than C v. This accords with our expectation, since energy will be consumed in expansion when a substance is heated at constant pressure, whereas this will not be the case for heating at constant volume. For an ideal gas, 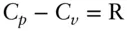 .
.
As it is impractical to measure C vfor solids and liquids, only experimentally determined values of C pare available for them, and values of C Vmust be obtained from eqn. 2.74when required.
We found earlier that C pis the variation of heat with temperature at constant pressure. How does this differ from the variation of energy with temperature at constant volume? To answer this question, we rearrange eqn. 2.71and substitute C Vfor ( ∂U/∂T) Vand Vα for (∂V/∂T) P. After simplifying the result, we obtain (on a molar basis):
(2.75) 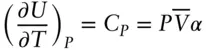
For an ideal gas, the term PVα reduces to R, so that ( ∂U / ∂T ) P= C p− R. C p− R may be shown to be equal to C V, so the energy change with temperature for an ideal gas is the same for both constant pressure and constant volume conditions. This is consistent with the notion that the difference between C pand C vreflects the energy associated with, and changing distances between, atoms and molecules in the presence of attractive forces between them. In an ideal gas, there are no such forces, hence 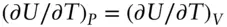 .
.
2.8.4 Heat capacity of solids: a problem in quantum physics
As we shall see, knowledge of the heat capacity of substances turns out to be critical to determining properties such as enthalpy and entropy, and, ultimately, to predicting chemical equilibrium. The heat capacity of a substance reflects the internal motion of its atoms. There are three kinds of motion available to atoms and molecules: translational, vibrational and rotational, †but often one or more of these modes will not be available and not contribute to the energy of a substance. For gases at low temperature, only rotational and translational motions are important (for a monatomic gas, only translational modes are available), while only vibrational motions are important for solids (translational modes are available to solids, which is why solids have finite vapor pressures, but they are extremely improbable, which is why vapor pressures of solids are very small and can usually be neglected). Twice as much energy is typically required to raise the temperature of a vibrational mode by 1 K as for a translational mode. This is because vibration involves both kinetic and potential energy of two or more atoms. Also, vibrational modes do not accept much energy at low temperatures.
This latter phenomenon is not predicted by classical physics; as a result, nineteenth-century physicists were puzzled by the temperature dependence of heat capacity. In 1869, James Maxwell referred to the problem as “the greatest difficulty yet encountered in molecular theory.” The solution required a more radical revision to physics than Maxwell imagined: the heat capacity problem turned out to be one of the first indications of the inadequacy of classical physics.
An understanding of the dependence of heat capacity on temperature was only achieved in the twentieth century with the aid of quantum physics. A complete theoretical treatment of heat capacity of real substances is beyond the scope of this book. However, even the few statements we will make will require us to make another excursion into statistical mechanics, a closely related field, to discover the Boltzmann distribution law. What we learn will be of considerable use in subsequent chapters.
2.8.4.1 The Boltzmann distribution law
Consider a mineral sample, A, in a heat bath, B (B having much more mass than A), and assume they are perfectly isolated from their surroundings. The total energy of the system is fixed, but the energy of A and B will oscillate about their most probable values. The question we ask is what is the probability that system A is in a state such that it has energy E A ?
We assume that the number of states accessible to A when it has energy E Ais some function of energy:
(2.76) 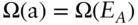
Following the basic postulate, we also assume that all states are equally probable and that the probability of a system having a given energy is simply proportional to the number of states the system can assume when it has that energy:
(2.39) 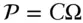
where C is a constant. Thus, the probability of A being in state a with energy E Ais:
(2.77) 
Since the total energy of the two systems is fixed, system B will have some fixed energy E Bwhen A is in state a with energy E A,and:
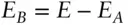
where E is the total energy of the system. As we mentioned earlier, Ω is multiplicative, so the number of states available to the total system, A + B, is the product of the number of states available to A times the states available to B:
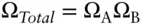
If we stipulate that A is in state a , then Ω Ais 1 and the total number of states available to the system in that situation is just Ω B:
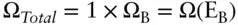
Thus, the probability of finding A in state a is equal to the probability of finding B in one of the states associated with energy E B, so that:
(2.78) 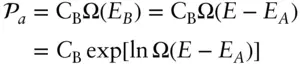
We can expand 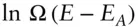 as a Taylor series about E:
as a Taylor series about E:
(2.79) 
and since B is much larger than A, E > E A, higher-order terms may be neglected.
Читать дальше


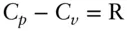 .
.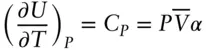
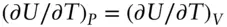 .
.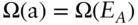
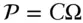

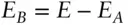
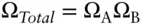
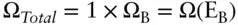
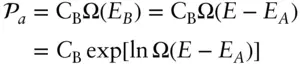
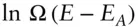 as a Taylor series about E:
as a Taylor series about E:











