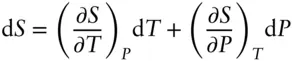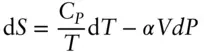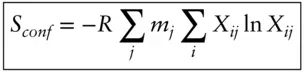Nevertheless, geochemists generally use empirically determined heat capacities. Constant pressure heat capacities are easier to determine, and therefore more generally available and used. For minerals, which are relatively incompressible, the difference between C vand C pis small and can often be neglected. Empirical heat capacity data is generally in the form of the coefficients of polynomial expressions of temperature. The Maier-Kelley formulation is:
(2.102) 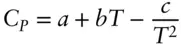
where a , b , and c are the empirically determined coefficients. The Haas–Fisher formulation (Hass and Fisher, 1976) is:
(2.103) 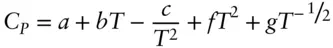
with a , b , c , f , and g as empirically determined constants. The Hass–Fisher formulation is more accurate and more widely used in geochemistry and heat capacity data are commonly tabulated this way (e.g., Helgenson, et al., 1978; Berman, 1988; Holland and Powell, 1998). We shall use the Maier−Kelly formulation because it is simpler, and we do not want to become more bogged down in mathematics than necessary.
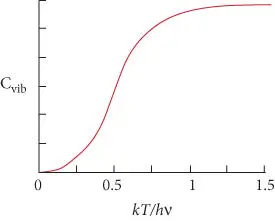
Figure 2.10 Vibrational contribution to heat capacity as a function of k T / hν .
Since these formulae and their associated constants are purely empirical (i.e., neither the equations nor constants have a theoretical basis), they should not be extrapolated beyond the calibrated range.
2.8.5 Relationship of entropy to other state variables
We can now use heat capacity to define the temperature dependency of entropy:
(2.104) 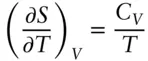
(2.105) 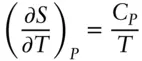
The dependencies on pressure and volume (at constant temperature) are:
(2.106) 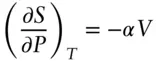
(2.107) 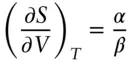
2.8.6 Additive nature of silicate heat capacities
For many oxides and silicates, heat capacities are approximately additive at room temperature. Thus, for example, the heat capacity of enstatite, MgSiO 3,, may be approximated by adding the heat capacities of its oxide components, quartz (SiO 2) and periclase (MgO). In other words, since:
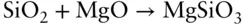
then
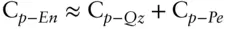
Substituting values:
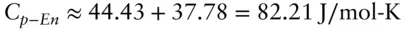
The observed value for the heat capacity of enstatite at 300 K is 82.09 J/mol-K, which differs from our estimate by only 0.1%. For most silicates and oxides, this approach will yield estimates of heat capacities that are within 5% of the observed values. However, this is not true at low temperature. The same calculation for C p-Encarried out using heat capacities at 50 K differs from the observed value by 20%.
The explanation for the additive nature of oxide and silicate heat capacities has to do with the nature of bonding and atomic vibrations. The vibrations that are not fully activated at room temperature are largely dependent on the nature of the individual cation–oxygen bonds and not on the atomic arrangement in complex solids.
2.9 THE THIRD LAW AND ABSOLUTE ENTROPY
2.9.1 Statement of the third law
The entropies of substances tend toward zero as absolute zero temperature is approached, or as Lewis and Randall expressed it:
If the entropy of each element in some crystalline state may be taken as zero at the absolute zero of temperature, every substance has a finite positive entropy, but at absolute zero, the entropy may become zero, and does so become in the case of perfectly crystalline substances .
We recall that entropy is proportional to the number of possible arrangements of a system: S = klnΩ. At absolute zero, a perfectly crystalline substance has only one possible arrangement, namely the ground state. Hence 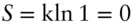 .
.
The implication of this seemingly trivial statement is that we can determine the absolute entropy of substances. We can write the complete differential for S in terms of T and P as:
(2.108) 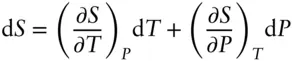
Substituting eqns. 2.105and 2.106into this, we have:
(2.109) 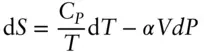
The coefficient of thermal expansion is 0 at absolute zero; the choice of 1 atm for the heat capacity integration is a matter of convenience because C Pmeasurements are conventionally made at 1 atm.
Actually, the absolute entropies of real substances tend not to be zero at absolute zero, which is to say they are not “perfectly crystalline” in the third law sense. A residual entropy, S 0, which reflects such things as mixing of two or more kinds of atoms (elements or even isotopes of the same element) at crystallographically equivalent sites, must also be considered. This configurational entropy is important for some geologically important substances such as feldspars and amphiboles. Configurational entropy can be calculated as:
(2.110) 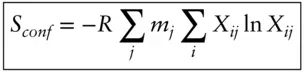
where m jis the total number of atoms in the j thcrystallographic site (in atoms per formula unit) and X i,jis the mole fraction of the i thatom (element) in the j thsite (see Example 2.3). We will return to this equation when we consider multicomponent systems.
Example 2.3Configurational entropy
Olivine is an example of a solid solution, which we will discuss at length in Chapter 3. Fe and Mg may substitute for each other in the octahedral site. Assuming that the distribution of Fe and Mg within this site is purely random, what is the configurational entropy of olivine of the composition (Mg 0.8,Fe 0.2) 2SiO 4?
Читать дальше

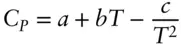
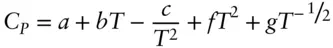

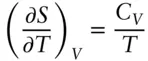
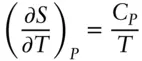
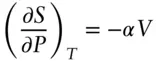
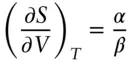
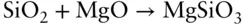
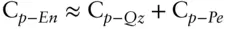
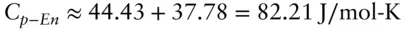
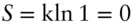 .
.