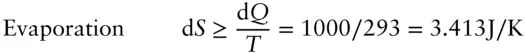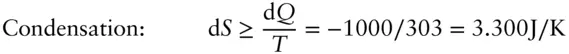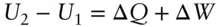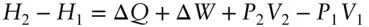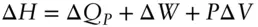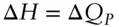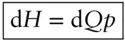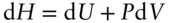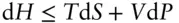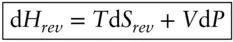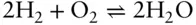By definition, an adiabatic system is one where d Q = 0. Since d Q rev/ T = d S rev( eqn. 2.52), it follows that for a reversible process, an adiabatic change is one carried out at constant entropy, or in other words, an isoentropic change . For adiabatic expansion or compression, therefore, d U = – P d V .
Example 2.1Entropy in reversible and irreversible reactions
Air conditioners work by allowing coolant contained in a closed system of pipes to evaporate in the presence of the air to be cooled, then recondensing the coolant (by compressing it) on the warm or exhaust side of the system. Let us define our “system” as only the coolant in the pipes. The system is closed since it can exchange heat and do work but not exchange mass. Suppose our system is contained in an air conditioner maintaining a room at 20°C or 293 K and exhausting to outside air at 303 K. Let's assume the heat of evaporation of the coolant (the energy required to transform it from liquid to gas) is 1000 joules. During evaporation, the heat absorbed by the coolant, d Q , will be 1000 J. During condensation, –1000 J will be given up by the system. For each cycle, the minimum entropy change during these transformations is easy to calculate from eqn. 2.51:
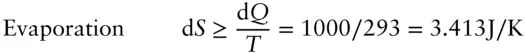
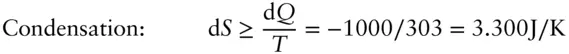
The minimum net entropy change in this cycle is the sum of the two, or 3.413 – 3.300 = 0.113 J/K. This is a “real” process and irreversible, so the entropy change will be greater than this.
If we performed the evaporation and condensation isothermally at the equilibrium condensation temperature (i.e., reversibly), then this result gives the exact entropy change in each case. In this imaginary reversible reaction, where equilibrium is always maintained, there would be no net entropy change over the cycle. But of course no cooling would be achieved either, so it would be pointless from a practical viewpoint. It is nevertheless useful to assume this sort of reversible reaction for the purposes of thermodynamic calculations, because exact solutions are obtained.
We have now introduced all the fundamental variables of thermodynamics, T, S, U, P and V . Everything else can be developed and derived from these functions. Thermodynamicists have found it convenient to define several other state functions, the first of which is called enthalpy . Enthalpy is a composite function and is the sum of the internal energy plus the product PV :
(2.59) 
As is the case for most thermodynamic functions, it is enthalpy changes rather than absolute enthalpy that are most often of interest. For a system going from state 1 to state 2, the enthalpy change is:
(2.60) 
The first law states:
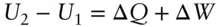
so:
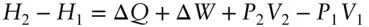
If pressure is constant, then:
(2.61) 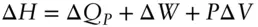
(we use the subscript P in ΔQ Pto remind us that pressure is constant). If the change takes place at constant pressure and P–V work is the only work done by the system , then the last two terms cancel and enthalpy is simply equal to the heat gained or lost by the system:
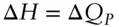
or in differential form:
(2.62) 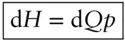
H is a state function because it is defined in terms of state functions U , P , and V . Because enthalpy is a state function, d Q must also be a state function under the conditions of constant pressure and the only work done being P – V work.
More generally, the enthalpy change of a system may be expressed as:

or at constant pressure as:
(2.63) 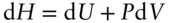
In terms of its characteristic variables, it may also be expressed as:
(2.64) 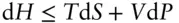
From this it can be shown that H will be at a minimum at equilibrium when S and P are prescribed:
(2.65) 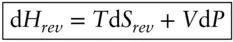
The primary value of enthalpy is measuring the energy consumed or released in changes of state of a system. For example, how much energy is given off by the reaction:
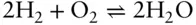
To determine the answer, we could place hydrogen and oxygen in a well-insulated piston-cylinder maintaining constant pressure. We would design it such that we could easily measure the temperature before and after reaction. Such an apparatus is known as a calorimeter . By measuring the temperature before and after the reaction and knowing the heat capacity of the reactants and our calorimeter, we could determine the enthalpy of this reaction. This enthalpy value is often also called the heat of reaction or heat of formation and is designated ΔH r(or ΔH ƒ). Similarly, we might wish to know how much heat is given off when NaCl is dissolved in water. Measuring temperature before and after reaction would allow us to calculate the heat of solution. The enthalpy change of a system that undergoes melting is known as the heat of fusion or heat of melting, ΔH m(this quantity is sometimes denoted Δ H f; we will use the subscript m to avoid confusion with heat of formation); that of a system undergoing boiling is known as the heat of vaporization, ΔH v. As eqn. 2.65suggests, measuring enthalpy change is also a convenient way of determining the entropy change.
At this point, it might seem that we have wandered rather far from geochemistry. However, we shall shortly see that functions such as entropy and enthalpy and measurements of such things as heats of solution and melting are essential to predicting equilibrium geochemical systems.
Читать дальше