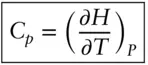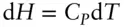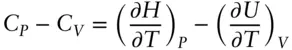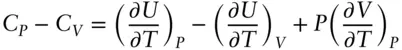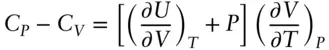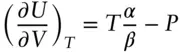Example 2.2Measuring enthalpies of reaction
Sodium reacts spontaneously and vigorously with oxygen to form Na 2O. The heat given off by this reaction is the enthalpy of formation ΔH ƒof Na 2O. Suppose that you react 23 g of Na metal with oxygen in a calorimeter that has the effective heat capacity of 5 kg of water. The heat capacity of water is 75.3 J/mol K. If the calorimeter has a temperature of 20°C before the reaction and a temperature of 29.9°C after the reaction, what is Δ H ƒof Na 2O? Assume that the Na 2O contributes negligibly to the heat capacity of the system.
Answer: The heat capacity of the calorimeter is
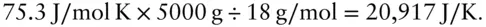
The heat required to raise its temperature by 9.9 K is then
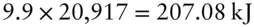
which is the enthalpy of this reaction. Our experiment created 0.5 moles of Na 2O, so ΔH is −414.16 kJ/mol.
It is a matter of everyday experience that the addition of heat to a body will raise its temperature. We also know that if we bring two bodies in contact, they will eventually reach the same temperature. In that state, the bodies are said to be in thermal equilibrium. However, thermal energy will not necessarily be partitioned equally between the two bodies. It would require half again as much heat to increase the temperature of 1 g of quartz by 1°C as it would to increase the temperature of 1 g of iron metal by 1°C. (We saw that temperature is a measure of the energy per degree of freedom. It would appear then that quartz and iron have different degrees of freedom per gram, something we will explore below.) Heat capacity is the amount of heat (in joules or calories) required to raise the temperature of a given amount (usually a mole) of a substance by 1 K. Mathematically, we would say:
(2.66) 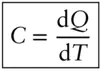
However, the heat capacity of a substance will depend on whether heat is added at constant volume or constant pressure, because some of the heat will be consumed as work if the volume changes. Thus, a substance will have two values of heat capacity: one for constant volume and one for constant pressure.
2.8.1 Constant volume heat capacity
Recall that the first law states:
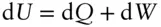
If we restrict work to P – V work, this may be rewritten as:
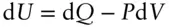
If the heating is carried out at constant volume (i.e., d V = 0), then d U = d Q (all energy change takes the form of heat) and:
(2.67) 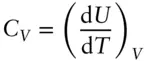
In an ideal gas, each atom has three degrees of translational freedom. A mole of such gas will have N Asuch atoms and 3N Adegrees of freedom. According to the kinetic theory of gases, the energy, U , of this gas is 3/ 2N Ak T . Thus (d U /d T ) V= 3/ 2N Ak, or 3/ 2R where R is the gas constant. Molecular gases, however, are not ideal. Vibrational and rotational modes also come into play, and heat capacity of real gases, as well as solids and liquids, is a function of temperature.
For solids, motion is vibrational and heat capacities depend on vibrational frequencies, which in turn depend on temperature and bond strength (for stronger bonds there is less energy stored as potential energy, hence less energy is required to raise temperature), for reasons discussed below. For nearly incompressible substances such as solids, the difference between C Vand C Pis generally small.
2.8.2 Constant pressure heat capacity
While heat capacities at constant volume are readily measured for gases, they are difficult to measure for solids and liquids. In nature too, temperature changes tend not to take place at constant volume, so constant pressure heat capacities are of greater interest. equation 2.61states that 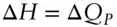 . Substituting this expression in to eqn. 2.66we have:
. Substituting this expression in to eqn. 2.66we have:
(2.68) 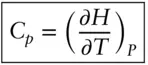
Thus, enthalpy change at constant pressure may also be expressed as:
(2.69) 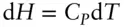
2.8.3 Energy associated with volume and the relationship between C vand C p
Constant pressure and constant temperature heat capacities are different because there is energy associated (work done) with expansion and contraction. Thus how much energy we must transfer to a substance to raise its temperature will depend on whether some of this energy will be consumed in this process of expansion. These energy changes are due to potential energy changes associated with changing the position of an atom or molecule in the electrostatic fields of its neighbors. The difference between C Vand C preflects this energy associated with volume. Let us now determine what this difference is.
We can combine relations 2.67and 2.68as:
(2.70) 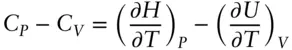
From this, we may derive the following relationship:
(2.71) 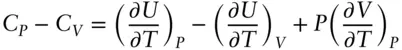
and further:
(2.72) 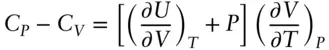
It can also be shown that, for a reversible process:
(2.73) 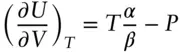
( ∂ U/ ∂ V) Tis the energy associated with the volume occupied by a substance and is known as the internal pressure ( P int, which we introduced earlier in our discussion of the van der Waals law, e.g., eqn. 2.17). It is a measure of the energy associated with the forces holding molecules or atoms together. For real substances, energy changes associated with volume changes reflect potential energy increases associated with increased separation between charged molecules and/or atoms; there are no such forces in an ideal gas, so this term is 0 for an ideal gas. Substituting eqn. 2.73into 2.72, we obtain:
Читать дальше

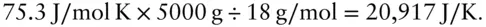
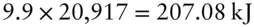
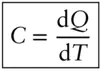
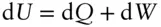
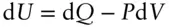
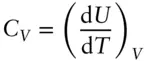
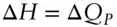 . Substituting this expression in to eqn. 2.66we have:
. Substituting this expression in to eqn. 2.66we have: