The import of membrane proteinsis similar. The growing polypeptide chain is internalized until a second signal sequence, which corresponds to a transmembrane domain, is reached ( Figure 5.6). The cleavage of the first signal sequence results in a transmembrane protein with a transmembrane region. The C‐terminal lies in the cytosol and the N‐terminal in the ER lumen. The formation of membrane proteins with many transmembrane regions occurs in a similar way. Some proteins (including SNARE) are anchored in the ER membrane by a C‐terminal hydrophobic α‐helix.
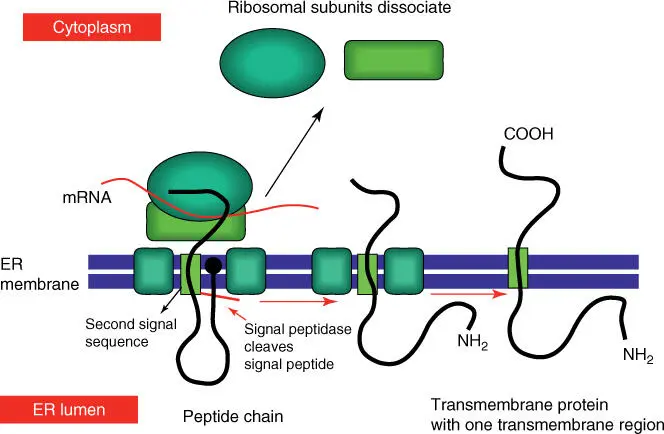
Figure 5.6 Simplified scheme of the integration of a membrane protein into the ER membrane.
Proteins that remain in the ER and are not channeled out through the Golgi apparatus have a retention signal at the C‐terminal. Such ER proteins serve, among others, as chaperones. Misfolded proteins are exported into the cytosol where they are degraded by the proteasome.
Upon entry into the ER, most proteins that are to be exported are coupled with an oligosaccharide residue. An oligosaccharide is linked to an asparagine residuevia an N‐glycosidic bond. Oligosaccharides with 14 sugar residues (above all those containing N ‐acetylglucosamine, mannose, and glucose) are present as dolichol diphosphate estersin the activated form, in which the lipophilic dolichol residue is anchored in the biomembrane ( Figure 5.7). Also present in the cell are glycoproteins whose sugar residues are linked to threonine or serine with an O ‐glycosidic bond. Their synthesis occurs in the Golgi apparatusand not in the ER. The sugar residues are altered again in the different compartments of the Golgi apparatus, where they obtain their final specificity.
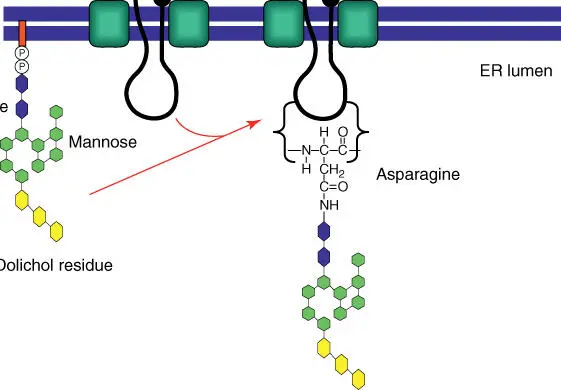
Figure 5.7 Assembly of glycoproteins in the ER. The oligosaccharide exists as a dolichol diphosphate ester in its activated form and can be transferred onto an asparagine residue of the growing peptide chain.
A few proteins are associated with the cell membrane. This usually occurs through a glycosylphosphatidylinositol ( GPI ) anchor, which can be attached to the C‐terminal of a protein.
5.4 Vesicle Transport from the ER via the Golgi Apparatus to the Cytoplasmic Membrane
The endomembrane systemof the cell shows a high degree of dynamics through the uptake and secretion of vesicles. Proteins from the ER are also transported in this way to the Golgi apparatusand from the Golgi apparatus to the lysosomesand endosomesas well as the cytoplasmic membrane( Figure 5.8).
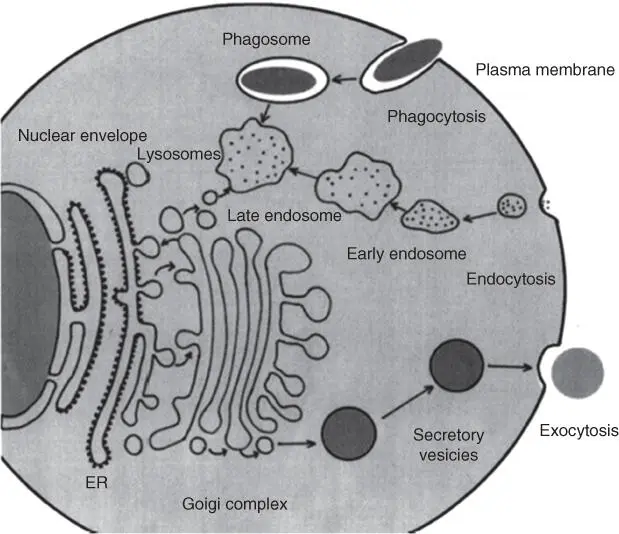
Figure 5.8 Vesicle transport pathways in the cell.
The pinching off of vesiclesand their uptake is a complex process that involves a large number of internal and external proteins (many of them not yet known). The budding of vesiclesonly occurs when a specific protein coat is formed on the vesicle surface:
Vesicles that bud from the ER carry COPII proteins.
Vesicles that migrate between the cis and trans sides of the Golgi apparatus carry COPI proteins.
Vesicles that are sent from the cis‐Golgi to the endosomes or endocytotic vesicles from the plasma membrane are covered with a coat of clathrin molecules ( Figure 5.9).
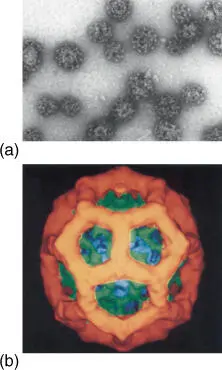
Figure 5.9 Structure of clathrin‐coated vesicles: (a) electron micrograph and (b) three‐dimensional representation of a clathrin coat, derived from an electron microscope photo.
Source: Courtesy of Barbara Pearse, Medical Research Council, Cambridge, UK.
These surface proteins are connected to membrane‐bound cargo receptors via adapter proteins, which recognize cargo proteins that are present within the vesicle.
Vesicles must be able to recognize a target compartment and to bring the content to the correct location. Further receptor molecules termed SNARE proteinsserve this purpose. Every vesicle carries specific v‐SNAREproteins on the surface, which can be recognized by the target compartment with specific t‐SNARE receptors. In this context, Rab proteinsare important (Table 5.2): Rab proteins are monomeric GTPases that with the help of other proteins ( Rab cascade) ensure that the vesicle finds the right partner.
Table 5.2 Occurrence of some Rab proteins.
| Rab |
Localization |
| Rab1 |
ER and Golgi |
| Rab2 |
cis‐Golgi network |
| Rab3A |
Synaptic and secretory vesicles |
| Rab4/Rab11 |
Recycling endosomes |
| Rab5 |
Early endosomes, clathrin‐coated vesicles |
| Rab6 |
Medial and trans ‐ Golgi |
| Rab7 |
Late endosome |
| Rab8 |
Cilia |
| Rab9 |
Late endosomes, trans‐Golgi |
The most researched SNARE proteins are those associated with the neurovesiclesin the presynapse. Neurovesicles can only carry out exocytosis when synaptobrevin(v‐SNARE) on the vesicle membrane interacts with syntaxin(t‐SNARE) on the inside of the presynapse. Additionally a further peripheral membrane protein, SNAP25(t‐SNARE), must enter the complex. The exocytosis is initiated via a calcium signal: when an action potentialoccurs in the synapse, the voltage‐gated calcium channels open, and Ca 2+flows into the synapse for a short time.
In the different compartments of the Golgi apparatus, the sugar residues of the proteins are altered in different ways. For example, the mannose residuesof the lysosomal proteinsare phosphorylated and therefore recognized by their mannose‐6‐phosphate residues. In other proteins, the mannose residues are removed and replaced by N ‐acetylglucosamine, galactose, or N ‐acetylneuraminic acid (NANA).
In the trans‐Golgi, proteins with mannose‐6‐phosphate residuesare recognized by a specific transmembrane receptor. The loading of these receptors results in a conformation change in the proteins, which is then recognized by clathrin molecules( Figure 5.9). This leads to the budding of the vesicle, which is loaded with lysosomal enzymes. These vesicles fuse with vesicles of the late endosomes, finally resulting in the formation of the endosomes and lysosome.
Proteins that are sent to the cytoplasmic membrane, where they bud into the extracellular space via exocytosis, are also processed in the Golgi apparatus. The fusion of the Golgi vesicle with the cytoplasmic membrane is termed exocytosis. In this process, water‐soluble proteins, such as peptide hormonesor antibodies, are released into the extracellular space (e.g. the blood). Membrane‐associated proteins remain as membrane proteins in the cytoplasmic membrane and are orientated with their sugar residues into the extracellular space. Exocytosis can be both continuous and signal controlled. An example of the latter is the release of insulinor histaminefrom their respective storage vesicles.
Читать дальше







![Andrew Radford - Linguistics An Introduction [Second Edition]](/books/397851/andrew-radford-linguistics-an-introduction-second-thumb.webp)








