| Substrate |
Reaction conditions |
Nitroalkane |
Yield (%), (ee) |
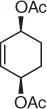 |
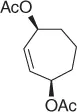
CH 3NO 2/CH 2Cl 2, Pd 2(dba) 3, ligand, BSA |
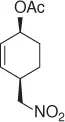 |
82 (99%) |
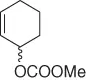 |
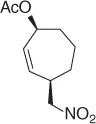
CH 3NO 2/CH 2Cl 2, Pd 2(dba) 3, ligand, Bu 4NCl/BSA |
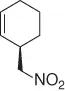 |
99 (99%) |
 |
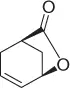
CH 3NO 2/CH 2Cl 2, Pd 2(dba) 3, ligand, BSA |
 |
84 (99%) |
 |
CH 3NO 2/CH 2Cl 2, Pd 2(dba) 3, ligand, Bu 4NCl/BSA |
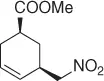 |
74 (99%) |

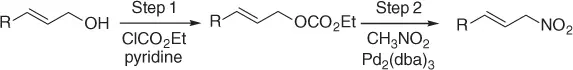
Scheme 1.20 Nitroalkanes addition to vinylepoxides under Pd(0) catalysis.
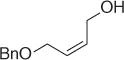
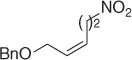
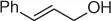
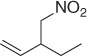
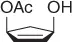
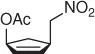
Table 1.7 Two-step process of nitro alkylation of allylic alcohols (selected examples).
 |
| Allylic alcohol |
Nitroalkane |
Yield (%) |
 |
 |
62 |
 |
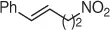 |
74 |
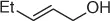 |
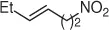 |
70 |
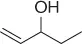 |
 |
71 |
 |
 |
63 |
Developing this result, Deardorff et al. [43] reported a two-step process for nitromethylation of allylic alcohols that include (i) the preparation of the corresponding allylic carbonate and (ii) the addition of nitromethane, under Pd 2(dba) 3catalysis.
So, this procedure represents a useful method for the synthesis of functionalized nitroalkanes and some of the most representative examples are reported in Table 1.7.
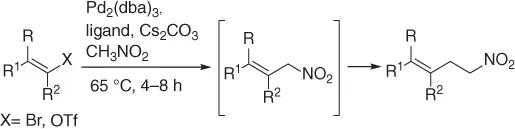
1.6.4 Two-Carbon Homologation of Vinyl Triflates and Bromides
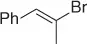
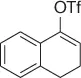
Palladium catalyzes a two-carbon homologation of vinyl bromides (or triflates) via reaction with nitromethane and in the presence of Cs 2CO 3as base, giving access to homoallylic nitrocompounds ( Table 1.8) [44].
The process exploits the anion stabilizing effect and the leaving properties of the nitro group to generate the homoallylic nitro products via a tandem cross-coupling/π-allylation sequence of the alkenyl derivatives with nitromethane. Following this method a variety of homoallylic nitro compounds can be easily obtained.
Table 1.8 Two carbon homologation of vinyl bromides or triflates (selected examples).
 |
| Substrate |
Nitroalkane |
Yield (%) |
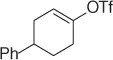 |
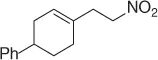 |
76 |
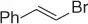 |
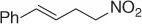 |
69 |
 |
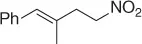 |
69 |
 |
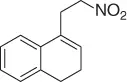 |
74 |
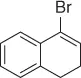 |
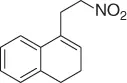 |
83 |
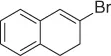 |
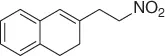 |
93 |
1 1 Ballini, R. and Palmieri, A. (2018). Adv. Synth. Catal. 360: 2240–2266.
2 2 Meyer, V. and Stuber, O. (1872). Ber. Dtsch. Chem. Ges. 5: 203–205.
3 3 Kornblum, N., Larson, H.O., Blackwood, R.K. et al. (1956). J. Am. Chem. Soc. 78: 1497–1501.
4 4 Easton, C.J., Xia, L., Pitt, M.J. et al. (2001). Synthesis: 451–458.
Читать дальше













































