In contrast with the nitration of aromatic hydrocarbons that can easily performed using nitric acid in the presence of sulfuric acid, the selective nitration of aliphatic hydrocarbons is very difficult due to the exceeding low reactivity of the latter.
Currently, nitration reactions of aliphatic hydrocarbons are carried out at fairly high temperature using nitrogen dioxide or nitric acid, thanks to a free radical process, involving C—H bond homolysis [38]. Often, under such temperature conditions higher alkanes undergo also cleavage of the C–C skeleton. Thus, Ishii and coworkers [39] developed a milder method for the nitration of light alkanes and alkyl side-chain aromatic compounds with NO 2and HNO 3under N -hydroxyphthalimide (NHPI) or N -acetoxyphtalimide (NAPI) catalysis ( Table 1.5).
1.6 Metal-Catalyzed Alkylation or Arylation of Nitroalkanes
Commercially available nitroalkanes can be used as precursors of more complex structures through their alkylation or arylation, performed under metal catalysis.
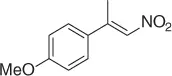
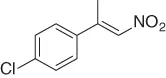
Table 1.4 Stereoselective reduction of nitroalkenes (selected examples).
 |
| Substrate |
Reducing system |
Yield (%), (ee) |
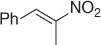 |
Baker’s yeast, EtOH·H 2O [33] |
91 ( S , 12%) |
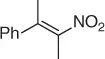 |
Baker’s yeast, EtOH·H 2O [33] |
72 ( S , 12%) |
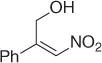 |
CuF 2/PhSiH 3, ( R )( S )-JOSIPHOS, PHMS/PhMe·H 2O [35] |
52 ( R , 90%) |
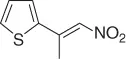 |
CuF 2/PhSiH 3, ( R )( S )-JOSIPHOS, PHMS/PhMe·H 2O [34] |
88 ( R 92%) |
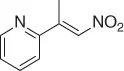 |
CuO- t -Bu/PhSiH 3, ( S )( R )-JOSIPHOS, PMHS/PhMe·H 2O [34] |
55 ( S , 72%) |
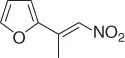 |
CuO- t -Bu/PhSiH 3, ( S )( R )-JOSIPHOS, PMHS/PhMe·H 2O [34] |
72 ( S , 90%) |
 |
Iridium complex, HCOOH/H 2O, pH 2 [34] |
94 ( R , 92%) |
 |
Iridium complex, HCOOH/H 2O, pH 2 [34] |
92 ( R , 90%) |
PMHS, polymethylhydrosiloxane.
1.6.1 Nitroalkylation of Aryl Halides
Aryl halides can be converted into “aryl nitromethanes” via a cross-coupling reaction with nitromethane, catalyzed by Pd 2(dba) 3, tris(dibenzylideneacetone)dipalladium, as reported in Scheme 1.18[40].
A more general protocol provides the arylation of primary nitroalkanes (different from nitromethane), via Pd-catalyzed reaction with aryl bromide or chlorides ( Scheme 1.19) [41].
More detailed applications are reported in Chapter 6.
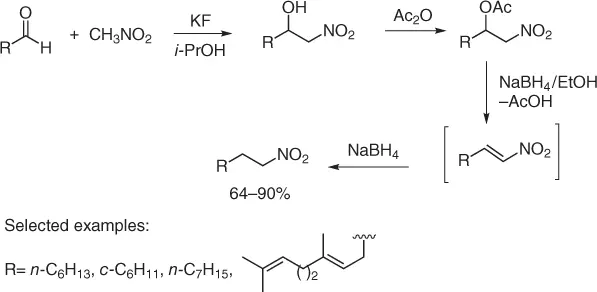
Scheme 1.17 Reductive nitromethylation of aldehydes.
Table 1.5 Nitration of alkanes (selected examples).
| Substrate |
Nitrating agent |
Nitroalkane |
Yield (%) |
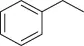 |
NHPI (NO 2) |
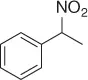 |
63 |
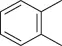 |
NAPI (HNO 3) |
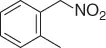 |
69 |
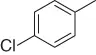 |
NAPI (NO 2) |
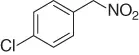 |
69 |
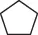 |
NHPI (HNO 3) |
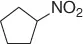 |
70 |
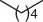 |
NHPI (NO 2) |
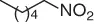 |
54 |
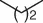 |
NHPI (NO 2) |
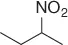 |
65 |
1.6.2 Nitroalkylation of Allylic Esters
Pd catalyzes even the asymmetric allylic alkylation of cyclic allyl esters [42] in the presence of O , N -bis-trimethylsilylacetamide (BSA) as base and a chiral biphosphine ligand ( Table 1.6). The method allows a practical way to primary chiral nitroalkanes with an extra carbon atom.
1.6.3 Nitroalkylation of Allylic Alcohols
Unactivated nitroalkenes (mainly nitromethane) can be added to vinyl epoxides, under Pd(0) catalysis, affording nitroalkenols ( Scheme 1.20).
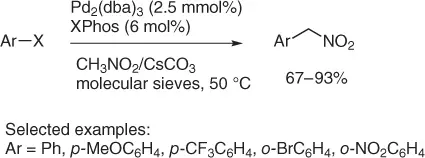
Scheme 1.18 Nitromethylation of aryl halides.
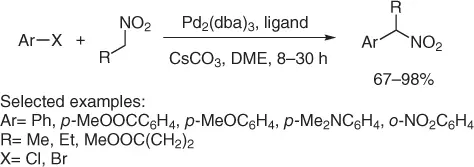
Scheme 1.19 Nitroalkylation of aryl halides.
Table 1.6 Asymmetric nitromethylation of cyclic allyl esters (selected examples).
Читать дальше