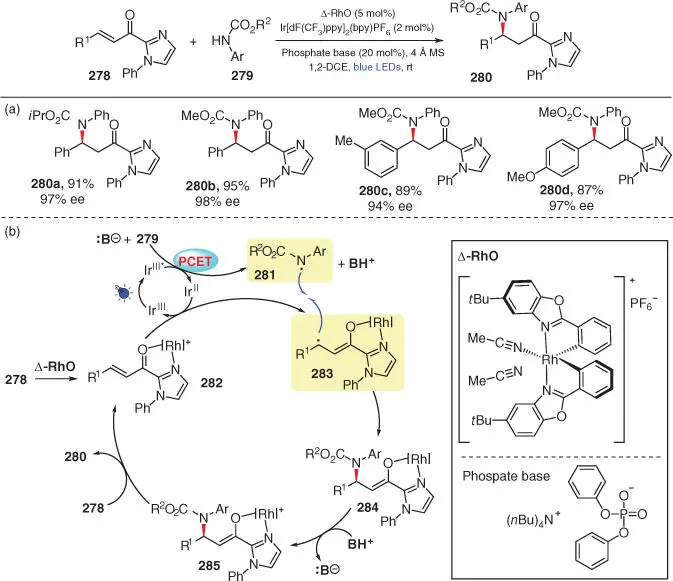
Scheme 3.46 Enantioselective amination via PCET followed by stereo‐controlled radical cross‐coupling.
Source: Modified from Zhou et al. [60].
3.5 Summary and Conclusions
This chapter focuses on the recent progress in direct C —N bond construction from unfunctionalized C–H and N–H partners via photo‐ or electrochemical methods. The reactions highlighted feature higher atom and step economy compared with the well‐established cross‐dehydrohalogenative couplings between C —H and N —X or C —X and N —H bonds. Amino functionalities, ranging from secondary to primary amines, are efficiently inserted into various C‐centered moieties. Particularly, the conceptually literal CDC amination, providing the amination products with the evolution of H 2gas, has been achieved by dual photocatalytic systems as well as catalyst‐free electrochemical methods without the requirement for any external oxidant. Furthermore, as summarized in this chapter, the radical intermediates engaged in these protocols have expanded the mechanistic patterns to N —H bonds nucleophilic addition, radical cross‐coupling, and others such as HAT, which are beyond the familiar N‐radical species addition.
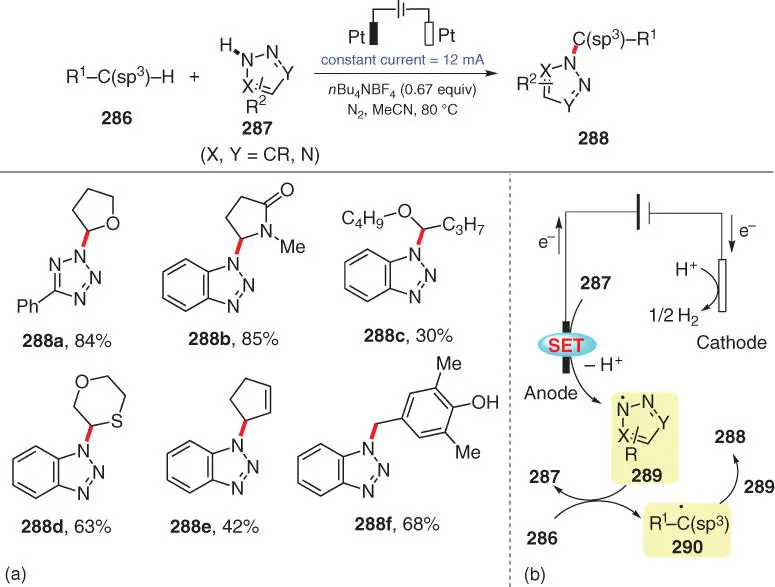
Scheme 3.47 Electrochemical intermolecular oxidative C(sp 3)–H/N–H cross‐coupling.
Source: Modified from Wu et al. [61].
Although an array of notable achievements has already been advanced in the recent years, there still remain certain challenges/opportunities in this field: (i) The N–H sources are generally limited to azoles, sulfonamides, secondary amides, carbamates, and diaryl amines. Only few examples have been developed that make use of primary amines or secondary amines. Therefore, developing a versatile strategy with a broader amine scope is still highly desirable; (ii) the direct amination of C(sp 3)–H has only been realized for the aliphatic ones on the activated sites such benzylic or α‐positions to a heteroatom, and the HAT‐facilitated amination is mostly limited to the functionalization at the remote δ‐H to a nitrogen substitution. The exploration of more versatile C(sp 3)–H aminations is also very attractive; (iii) highly enantioselective aminations as well as site‐specific aminations on general aromatic rings are still rarely achieved in the simple radical‐involved processes, which needs further investigation.
In the near future, further efforts in this area may focus on the development of new catalysts or combined catalytic systems to broaden the amination generality, both in photo‐ and electrochemical protocols. Comprehensive mechanistic investigations are also necessary for the elucidation of the activation mode of the direct aminations, which can provide a better understanding of the reaction pathway and conversely inspire the design of more elegant reactions with a broader scope. Moreover, application of chiral catalysts to coordinate with the radical intermediates or directing groups installed on the substrates would potentially address the issue of enantioselectivity. Further disclosures are anticipated to make the direct amination strategy one of the most valuable approaches for C —N bond construction.
1 1 Shin, K., Kim, H., and Chang, S. (2015). Accounts of Chemical Research 48: 1040–1052.
2 2 Louillat, M.L. and Patureau, F.W. (2014). Chemical Society Reviews 43: 901–910.
3 3 Kim, H. and Chang, S. (2016). ACS Catalysis 6: 2341–2351.
4 4 Park, Y., Kim, Y., and Chang, S. (2017). Chemical Reviews 117: 9247–9301.
5 5 Selected example and references cited therein:Manna, S., Serebrennikova, P.O., Utepova, I.A. et al. (2015). Organic Letters 17: 4588–4591.
6 6 Xiong, T. and Zhang, Q. (2016). Chemical Society Reviews 45: 3069–3087.
7 7 Chen, J.R., Hu, X.Q., Lu, L.Q., and Xiao, W.J. (2016). Chemical Society Reviews 45: 2044–2056.
8 8 Kärkäs, M.D. (2017). ACS Catalysis 7: 4999–5022.
9 9 Gentry, E.C. and Knowles, R.R. (2016). Accounts of Chemical Research 49: 1546–1556.
10 10 Choi, G.J., Zhu, Q., Miller, D.C. et al. (2016). Nature 539: 268–271.
11 11 Chu, J.C.K. and Rovis, T. (2016). Nature 539: 272–275.
12 12 Zhu, Q., Graff, D.E., and Knowles, R.R. (2018). Journal of the American Chemical Society 140: 741–747.
13 13 Zhu, L., Xiong, P., Mao, Z.Y. et al. (2016). Angewandte Chemie, International Edition 55: 2226–2229.
14 14 Hou, Z.W., Mao, Z.Y., Zhao, H.B. et al. (2016). Angewandte Chemie, International Edition 55: 9168–9172.
15 15 Xiong, P., Xu, H.H., and Xu, H.C. (2017). Journal of the American Chemical Society 139: 2956–2959.
16 16 Hou, Z.W., Mao, Z.Y., Melcamu, Y.Y. et al. (2018). Angewandte Chemie, International Edition 57: 1636–1639.
17 17 Hu, X.Q., Chen, J.R., Wei, Q. et al. (2014). Angewandte Chemie, International Edition 53: 12163–12167.
18 18 Hu, X.Q., Qi, X., Chen, J.R. et al. (2016). Nature Communications 7: 11188.
19 19 Zhao, Q.Q., Hu, X.Q., Yang, M.N. et al. (2016). Chemical Communications 52: 12749–12752.
20 20 Hu, X.Q., Chen, J., Chen, J.R. et al. (2016). Chemistry ‐ A European Journal 22: 14141–14146.
21 21 Zhao, Q.Q., Chen, J., Yan, D.M. et al. (2017). Organic Letters 19: 3620–3623.
22 22 Brachet, E., Marzo, L., Selkti, M. et al. (2016). Chemical Science 7: 5002–5006.
23 23 Maity, S. and Zheng, N. (2012). Angewandte Chemie, International Edition 51: 9562–9566.
24 24 Morris, S.A., Nguyen, T.H., and Zheng, N. (2015). Advanced Synthesis and Catalysis 357: 2311–2316.
25 25 Musacchio, A.J., Nguyen, L.Q., Beard, G.H., and Knowles, R.R. (2014). Journal of the American Chemical Society 136: 12217–12220.
26 26 Musacchio, A.J., Lainhart, B.C., Zhang, X. et al. (2017). Science 355: 727–730.
27 27 Miller, D.C., Ganley, J.M., Musacchio, A.J. et al. (2019). Journal of the American Chemical Society 141: 16590–16594.
28 28 Parasram, M. and Gevorgyan, V. (2017). Chemical Society Reviews 46: 6227–6240.
29 29 Sagadevan, A., Ragupathi, A., Lin, C.‐C. et al. (2015). Green Chemistry 17: 1113–1119.
30 30 Tong, K., Liu, X., Zhang, Y., and Yu, S. (2016). Chemistry ‐ A European Journal 22: 15669–15673.
31 31 Yamaguchi, T., Yamaguchi, E., and Itoh, A. (2017). Organic Letters 19: 1282–1285.
32 32 Ito, E., Fukushima, T., Kawakami, T. et al. (2017). Chem 2: 383–392.
33 33 Margrey, K.A., Levens, A., and Nicewicz, D.A. (2017). Angewandte Chemie, International Edition 56: 15644–15648.
34 34 Zhao, H.B., Hou, Z.W., Liu, Z.J. et al. (2017). Angewandte Chemie, International Edition 56: 587–590.
35 35 Tang, S., Wang, S., Liu, Y. et al. (2018). Angewandte Chemie, International Edition 57: 4737–4741.
36 36 Song, C., Liu, K., Wang, Z. et al. (2019). Chemical Science 10: 7982–7987.
37 37 Romero, N.A., Margrey, K.A., Tay, N.E., and Nicewicz, D.A. (2015). Science 349: 1326–1330.
38 38 Niu, L., Yi, H., Wang, S. et al. (2017). Nature Communications 8: 14226.
39 39 Pandey, G., Singh, D., and Laha, R. (2017). Asian Journal of Organic Chemistry 6: 469–474.
40 40 Wang, J.H., Lei, T., Nan, X.L. et al. (2019). Organic Letters 21: 5581–5585.
41 41 Meyer, A.U., Berger, A.L., and König, B. (2016). Chemical Communications 52: 10918–10921.
Читать дальше











