Source: Modified from Xiong et al. [15].
A regiospecific electrochemical [3+2] annulation for the preparation of various imidazo‐fused N ‐heteroaromatic compounds was more recently described by Xu's group ( Scheme 3.7) [16]. Employing a novel tetra‐arylhydrazine ( 34) as the catalyst in a mixed solvent of acetonitrile and water, this electrosynthesis smoothly converts substrate 33into 35through a cyclization/C—N bond cleavage sequence and the release of one molecule of CO 2. Conversely, another product 36, wherein the carbonyl survived, could be obtained from certain substrates under another set of reaction conditions using THF/methanol as the solvent. Further attempts subjecting urea‐linked 37under the second conditions also resulted in the corresponding imidazopyridines 38with methoxycarbonyl substituents installed. The mechanism of this transformation is quite similar to the aforementioned ones, and the DFT studies reveal that the C—N bond‐forming pathway of 41ais both thermodynamically and kinetically favored over the alternative C=C bond‐forming pathway.
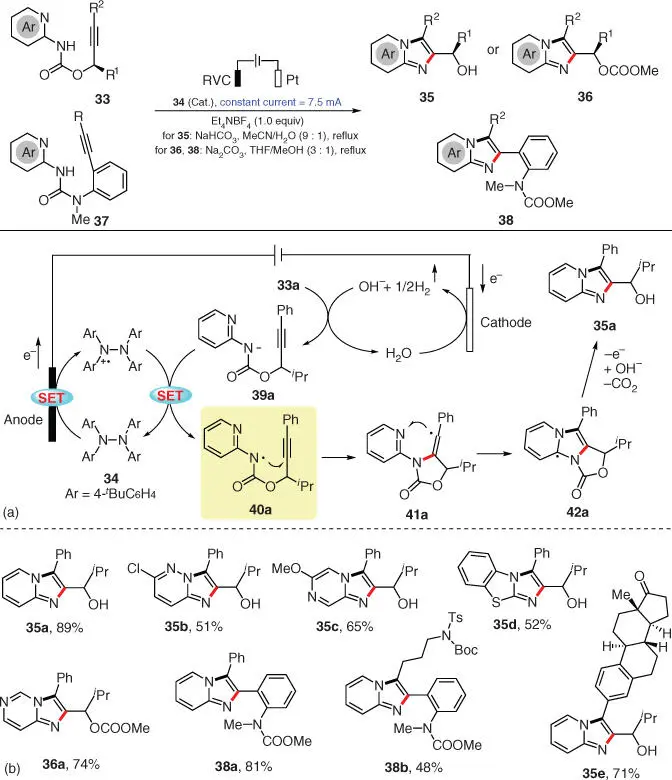
Scheme 3.7 Electrochemical synthesis of imidazo‐fused N‐heteroaromatic compounds.
Source: Modified from Hou et al. [16].
3.2.1.2 Hydrazonyl Radical Addition
Hydrazones are also competent N‐radical precursors under basic conditions. Since 2014, β,γ‐unsaturated hydrazone 43has been chosen by Xiao's group as a substrate to study a series of visible‐light‐induced intramolecular cyclization reactions, for the synthesis of various dihydropyrazole or dihydropyridazine derivatives ( Scheme 3.8a). Mechanistically, the acidic N—H bond of 43can be readily deprotonated by a base such as NaOH or K 2CO 3, and the resulting anionic species 44is prone to SET oxidation to generate the crucial radical intermediates 45. Next, there are two R 4‐dependent scenarios, 5‐exo‐trig or 6‐endo‐trig cyclization, for the further transformation of N‐radical 45to access the corresponding radical intermediate 46or 47, which can finally transform into diversified five‐ or six‐membered products after variable processes such as HAT, radical addition, etc.
The first work in this series is an intramolecular hydroamination of 43bearing terminal alkenes, which was developed in 2014 ( Scheme 3.8b) [17]. The desired product 48is produced from the five‐membered intermediate 46via a HAT process with the reaction solvent CHCl 3, which is demonstrated by a deuterium‐labeling experiment using CDCl 3as the solvent. Moreover, radical 46can also be intercepted by scavengers such as 2,2,6,6‐tetramethylpiperidine‐1‐oxyl (TEMPO) and diphenyltelluride (PhTeTePh) to form the corresponding adducts. In the second work published in 2016, the substituent R 4of 43is an aromatic group, instead of a hydrogen atom, giving access to 1,6‐dihydropyridazines 49under photoredox conditions, which is facilitated by a TEMPO‐mediated HAT process [18]. As calculated by DFT, the additional Ar‐group significantly lowers the activation free energy of a 6‐endo‐trig cyclization pathway and simultaneously stabilizes the formed intermediate 47. On the basis of these previous findings, Xiao and coworkers next set out to expand this N‐radical formation strategy of diversely substituted β,γ‐unsaturated hydrazones to other cascade transformations, including the synthesis of dihydropyrazole‐fused benzosultams 50through the addition of radical 46to the aromatic ring of the pendant –Ts group [19], the synthesis of –OH‐functionalized pyrazoline 51aor pyridazine 51bthrough trapping of oxygen by the radical intermediate 46or 47[20], and the synthesis of 52aor 52bvia the addition of radical 46or 47to allyl sulfones [21].
In 2016, another hydrazonyl radical‐based photoredox amination followed by a Smiles rearrangement was reported by Belmont's research group, wherein the N—H bond activation strategy is similar to that in Xiao's work ( Scheme 3.9) [22]. Phthalazine derivatives 54with varied substituents are obtained from alkynyl‐substituted sulfonyl hydrazones 53under basic conditions with the extrusion of SO 2( Scheme 3.9a). In light of the mechanistic studies including CV and fluorescence quenching experiments, it is proposed that the photoexcited catalyst Ru II* undergoes one‐electron reduction with anion 55, generated by deprotonation of substrate 53by the base NaOH. Subsequently, the produced N‐radical 56cyclizes onto its tethered alkyne to form vinylic radical 57, which is further engaged in a radical Smiles rearrangement to yield a delocalized radical species 58after the loss of one molecule of SO 2. After acquiring an electron from Ru Ispecies to complete the photoredox catalytic cycle, radical 58is reduced to anionic species 59, which is then protonated by the protic solvent EtOH to deliver the final product 54( Scheme 3.9b). Furthermore, the quantum yield of this reaction is measured as Φ = 0.33, which excludes a radical chain propagation process during the transformation. Additionally, attempts to realize one‐pot amination/Smiles rearrangement sequences by preparing 53in situ from the corresponding aldehydes and sulfonyl hydrazides also succeeded, with the same efficiency compared with the original two‐step protocol ( Scheme 3.9c).
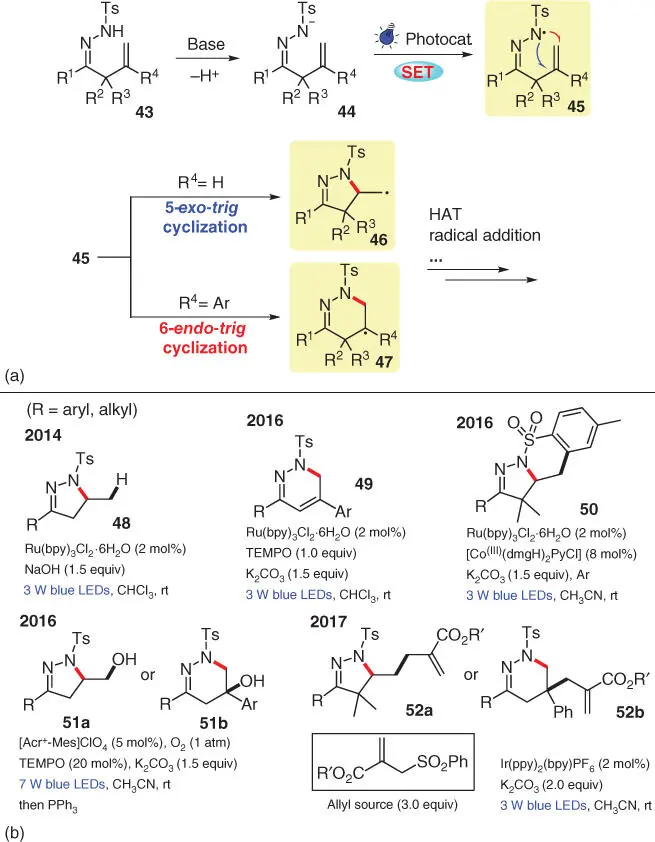
Scheme 3.8 Applying hydrazonyl radical for C—N bond formation in photochemistry.
Source: Modified from Hu et al. [17].
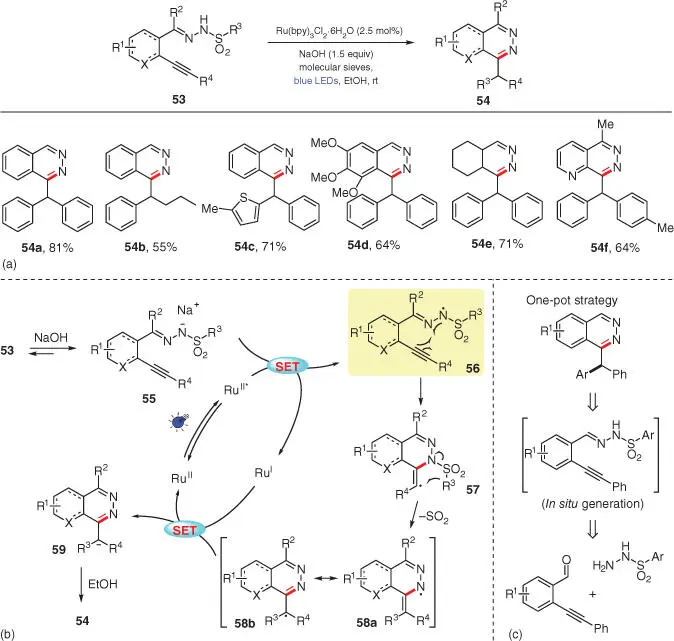
Scheme 3.9 Visible‐light‐enabled access to phthalazine derivatives via an amination/Smiles rearrangement cascade.
Source: Modified from Brachet et al. [22].
3.2.1.3 Aminium Radical Cation Addition
Electrophilic addition of aminium radical cations to unsaturated C=C bonds represents another attractive approach for the C—N bond construction. Generally, in visible‐light‐induced reactions, direct SET oxidation of the N—H bonds by the excited photocatalysts affords the crucial aminium radical cation intermediates, which are further converted into α‐amino radicals after addition to unsaturated C=C bonds to allow the C—N bond formation. In this field, representative work has been demonstrated independently by the research groups of Zheng and Knowles.
In 2012, Zheng's group disclosed a visible‐light‐induced intramolecular C—N bond formation/cyclization reaction of styryl diarylamines 60and 62to prepare N ‐arylindoles 61and 63under the irradiation of a white light‐emitting diode (LED) and ambient conditions ( Scheme 3.10) [23]. Interestingly, a 1,2‐carbon shift process is observed in the reaction of substrates 62with their C2 position fully occupied, affording R 1‐shifted indole products 63in moderate yields, wherein aryl groups are preferentially transferred over alkyl groups ( 63a). A reasonable reaction scope is presented, covering various substrates with electron‐donating or electron‐withdrawing substituents on Ar 1, as well as a wide range of substituents on C2 and C3 ( 61a– 61e). Moreover, the R 1‐migration process represents an elegant strategy for ring expansion ( 63b). Nevertheless, functional groups on Ar 2are limited to para ‐alkoxy ones to facilitate their effective oxidation.
Читать дальше












