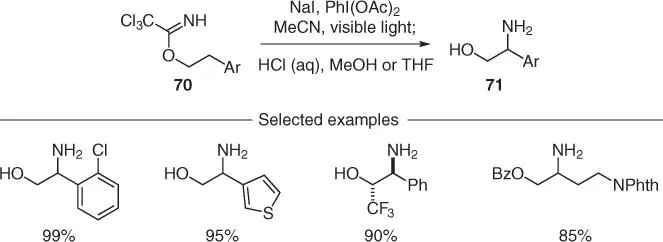
Scheme 2.17 Imidate radical mediated β‐C–H amination of alcohols.
Source: Modified from Wappes et al. [48].
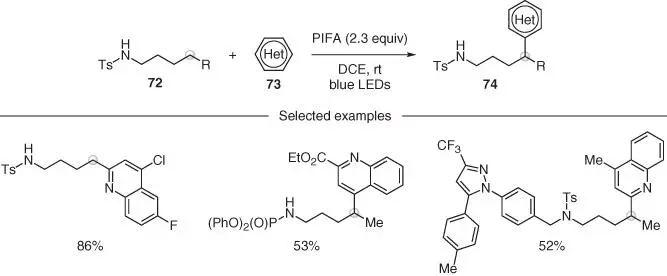
Scheme 2.18 Remote heteroarylation of amides via sulfonamidyl radicals [55].
Source: Gomez‐Suarez et al. [50], Hernandez et al. [51], Francisco et al. [52], Francisco et al. [53], and Martin et al. [54].
Alexanian and coworker developed a site‐specific C–H functionalization of amides using N ‐dithiocarbamates 75as precursors for amidyl radicals ( Scheme 2.19) [56]. Owing to the weak nature of N—S bonds, visible light irradiation (or standard radical initiation) enabled the generation of a nitrogen radical that underwent 1,5‐HAT, followed by xanthylation as part of a very efficient radical chain propagation. A wide range of complex molecules has been modified with this procedure, including complex natural products and pharmaceutical derivatives, thus showcasing the broad functional group compatibility.
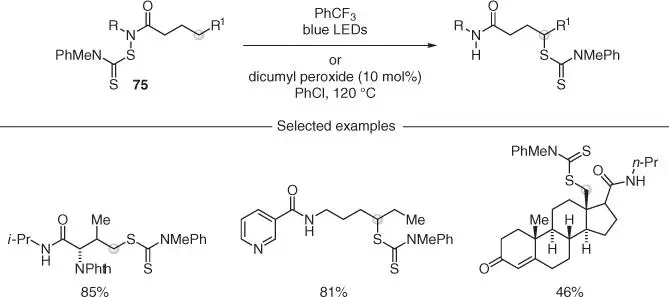
Scheme 2.19 Site‐specific C–H functionalization via HAT using N ‐dithiocarbamates.
Source: Modified from Na and Alexanian [56].
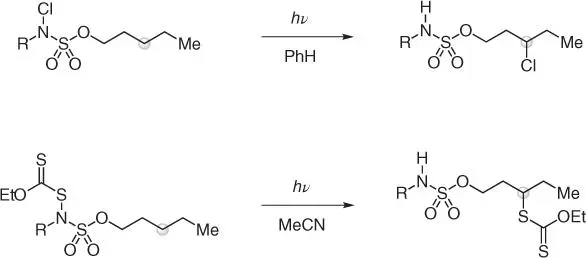
Scheme 2.20 γ‐C(sp 3)–H chlorination and xanthylation of sulfamate esters via 1,6‐HAT process.
Source: Short et al. [57] and Ayer and Roizen [58].
So far, all HAT strategies discussed have been mostly based on 1,5‐abstractions.The Roizen group has recently demonstrated how γ‐C(sp 3)–H chlorination and xanthylation of sulfamate esters is possible via a very selective 1,6‐process ( Scheme 2.20) [57, 58]. While this chemistry is mechanistically related to other procedures previously discussed, it has a peculiar selectivity because of the elongated N—S—O bond system that stabilizes a unique seven‐membered ring transition state.
The generation of nitrogen radicals and their utilization in radical transpositions is not restricted to photochemical approaches, and many powerful and applicable methods based on thermal processes have been reported.
Iminyl radicals can be conveniently accessed from electron‐poor O ‐acyl oximes by SET reduction as mentioned before (see Section 2.3.1.1). Similar starting materials 76have also been used in combination with Fe‐catalysis to access remote azidation of ketones ( Scheme 2.21) [59]. The proposed mechanism relied on an initial reduction of an Fe(III) species by TMSN 3to generate the active Fe(II) catalyst. This species was postulated to reduce the O ‐acyl oxime 76and give, after fragmentation, the corresponding iminyl radical 77. As these reactions were run under acidic conditions (AcOH), protonation of the iminyl radical would generate a more electrophilic species 78, thus reinforcing the polar effect and resulting in a more favorable 1,5‐HAT process. The resulting carbon radical 79was proposed to undergo an Fe(III)‐mediated azide transfer to give the product 80and regenerate the active Fe(II) catalyst. This methodology tolerated several functionalities including alkenes, heterocycles, nitriles, and substituted aromatics. Furthermore, by using N ‐acyloxy imidates, valuable β‐azido alcohols (after further hydrolysis) could also be obtained.
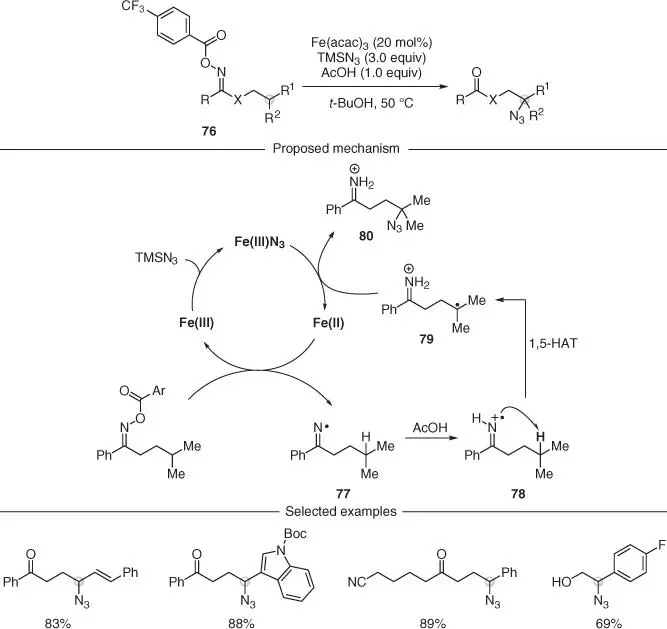
Scheme 2.21 Iron‐catalyzed remote C(sp 3)–H azidation of ketones via iminyl and imidate radicals.
Source: Modified from Torres‐Ochoa et al. [59].
Cook and coworkers reported the direct Csp 3–H fluorination of N –fluoroamides 81, employing Fe(OTf) 2as the catalyst ( Scheme 2.22) [60]. The mechanistic studies suggest that Fe(II) could directly cleave the N—F bond to generate an amidyl radical, which underwent nitrogen‐to‐carbon radical relay. Fe‐mediated fluorine transfer gave the products in generally high yields, also showing significant functional group tolerance.
Using a similar strategy, Zhu and coworkers designed a copper‐catalyzed variant, which allowed for a remote arylation using preformed N ‐fluoroamides 82( Scheme 2.23) [61]. Mechanistically, the authors proposed that Cu(I)‐mediated reductive cleavage of the amide N—F bond generated the amidyl radical 83, which underwent 1,5‐HAT from the benzylic position. Simultaneously, the Cu(II) species underwent transmetalation with the arylboronic acid 84, generating an Ar–Cu(II) species. This intermediate was intercepted by the carbon radical 85to give an Ar, alkyl–Cu(III)complex 86from which reductive elimination is facile. This step generated the desired arylated product 87along with the active Cu(I) catalyst. The methodology showed excellent functional group compatibility around the aromatic rings and could also be applied to heteroaromatic substrates. This Cu(I)/(II)/(III) manifold was also extended to the functionalization of nonbenzylic positions. However, these reactions generally gave lower yields owing to the increased flexibility of the chain when compared to the substrates with an embedded aromatic, which have a rigid and defined conformation.
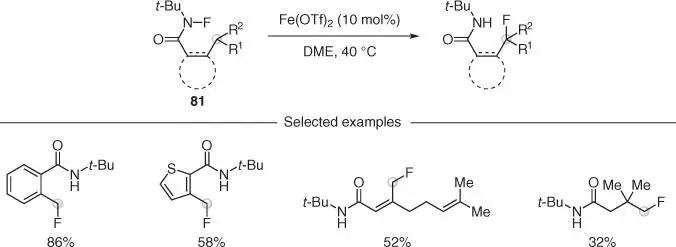
Scheme 2.22 Amide‐directed fluorination of C—H bonds catalyzed by iron.
Source: Modified from Groendyke et al. [60].
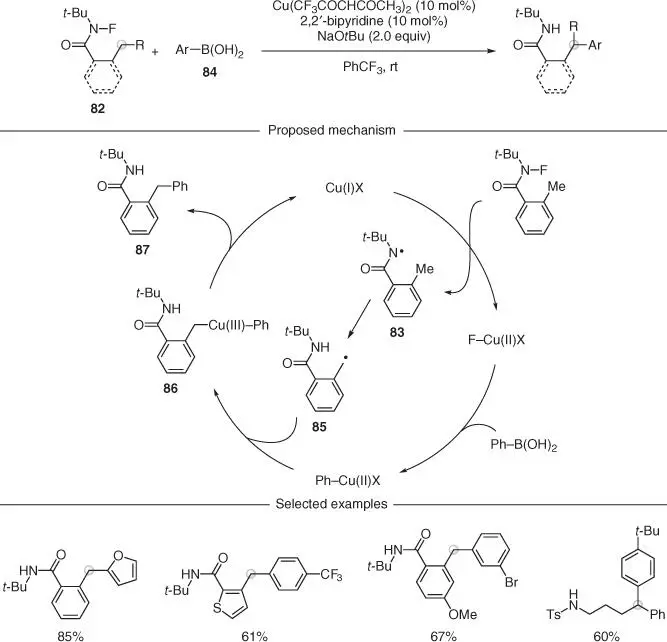
Scheme 2.23 Copper‐catalyzed remote arylation of C(sp 3)—H bonds via amidyl radicals.
Source: Modified from Li et al. [61].
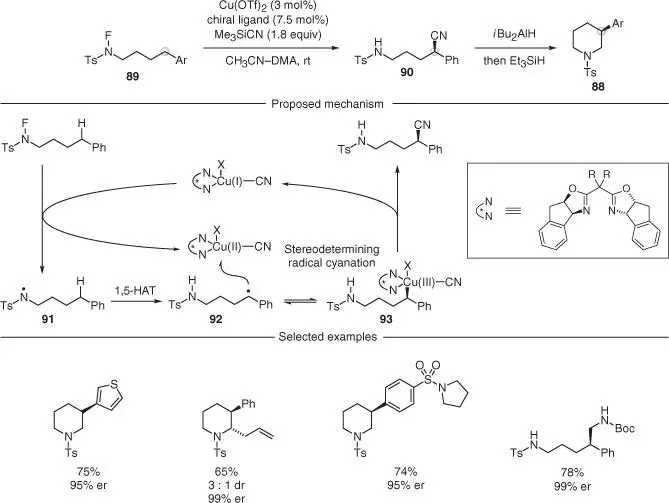
Scheme 2.24 Enantioselective remote C–H cyanation of amines via copper‐catalyzed radical relay.
Nagib and coworkers have recently provided a solution to the asymmetric synthesis of 3‐aryl substituted piperidines 88using an interrupted HLF reaction ( Scheme 2.24) [62]. By employing a Cu(I) catalyst in the presence of a chiral Box ligand, the authors achieved a rare example of enantioselective remote cyanation of N ‐fluoroamides 89in high yields and excellent enantioselectivities. These cyanated products 90were then converted in just two steps into the corresponding piperidines. The authors proposed a mechanism where an initial transmetalation between the Cu(I) catalyst and Me 3SiCN provided a Cu(I) intermediate capable of reducing the N—F bond in the amide starting materials. This step gave the amidyl radical 91that underwent N‐to‐C radical relay via 1,5‐HAT. The incipient carbon radical 92was intercepted by the Cu(II)CN species to give an alkyl–Cu(III)CN 93intermediate, from which a stereoselective reductive elimination provided the product and regenerated the Cu(I) catalyst. Although this chemistry is only compatible with the functionalization of benzylic positions, the authors applied it to the preparation of a range of cyanated substrates, all synthesized in good yields and enantioselectivities. Next, they subjected a number of products to a two‐step reduction with i ‐Bu 2AlH (to form a hemiaminal), followed by Et 3SiH to furnish the desired C‐3 arylated piperidine products.
Читать дальше

















