Source: Modified from Morcillo et al. [37].
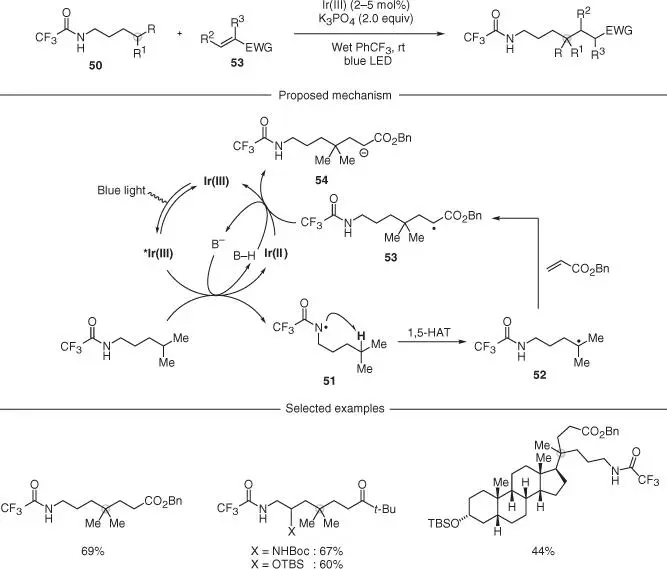
Scheme 2.13 Amide‐directed selective C—C bond formation at C(sp 3)—H bonds.
Source: Choi et al. [40] and Chu and Rovis [41].
This PCET reactivity for amidyl radical generation and 1,5‐HAT has also been exploited by Tamber to achieve remote amide allylation using allyl chlorides and a Ni(0) cocatalyst ( Scheme 2.14) [42].
A powerful expansion of this PCET strategy for amidyl radical generation and 1,5‐HAT has been recently introduced by Rovis and coworkers, who merged the photoredox manifold with nickel catalysis ( Scheme 2.15) [43]. This dual catalytic process used alkyl bromides as coupling partners and enabled a site‐selective C–H alkylation delivering products 55. Mechanistically, the trifluoroacetic acid (TFA)‐amide was oxidized through PCET by the excited Ir(III) catalyst upon visible light irradiation to form the amidyl radical 56. This species underwent 1,5‐HAT, delivering the carbon radical 57. At the same time, oxidative addition of a nickel(I) complex 58to the alkyl bromide resulted in an alkyl–Ni(III) complex 59that was proposed to undergo SET with the reduced photocatalyst. This event would close the photoredox cycle and generate an alkyl–Ni(II) species 60that could be intercepted by 57. This radical transmetalation enabled access to a dialkyl–Ni(III) intermediate 61from which reductive elimination is facile. This final step would forge the key sp 3–sp 3C—C bond and close the nickel cycle. Although this reactivity is restricted to the use of primary alkyl bromides as coupling partners, the authors demonstrated its ability to construct a broad range of remotely alkylated products.
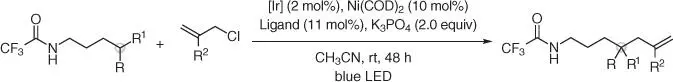
Scheme 2.14 Remote amide allylation using allyl chlorides and Ni(0) cocatalyst.
Source: Modified from Xu and Tambar [42].
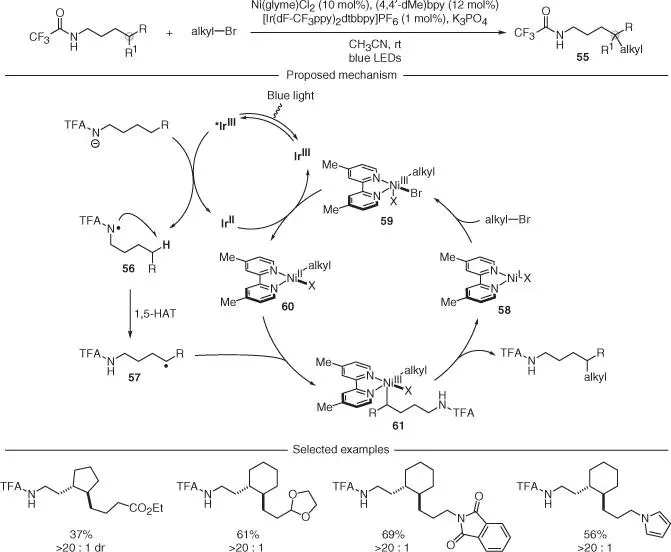
Scheme 2.15 Regioselective cross‐coupling of C(sp 3)—H bonds and alkyl bromides under a combination of photoredox and nickel catalysis.
2.3.3 Photoinduced Bond Homolysis
The weak nature of N—X (X = halogen) bonds has historically been of fundamental importance for the development of HLF reactions. However, the requirement for prior synthesis of N ‐iodo or N ‐bromo amines, which are sometimes unstable, represented a significant limitation.
In a series of elegant reports Muñiz and coworkers developed conditions for HLF conversion of unfunctionalized N ‐Ts amines 62into the corresponding pyrrolidines 63by using substoichiometric amounts of I 2and the I(III) reagent PhI( m CBA) 2as the oxidant ( Scheme 2.16) [44–46]. These processes are believed to be initiated by the reaction of I 2with PhI( m CBA) 2that leads to the formation of I( m CBA) 64, the active catalyst. Following detailed mechanistic studies, the authors suggested that this species promoted the in situ formation of an N ‐iodo– N ‐Ts amine 65, which underwent facile N—I bond homolysis upon visible light irradiation. Following 1,5‐HAT from the sulfonyl radical 66, the distal carbon radical 67was iodinated to give 68as part of a radical‐chain propagation. In classical HLF strategies, a basification is required to trigger the ionic cyclization; however, in this case, the PhI( m CBA) 2was able to directly oxidize 68to the corresponding I(III) intermediate 69. The very activated nature of this species means that an immediate nucleophilic displacement was possible, leading to the assembly of the desired N‐heterocycle. This step would also regenerate the active I( m CBA) catalyst. The authors have showcased this reactivity mostly for the functionalization of benzylic centers and the preparation of pyrrolidines. However, careful juxtaposition of substituents has also enabled access to the six‐membered ring (piperidine) analogs.
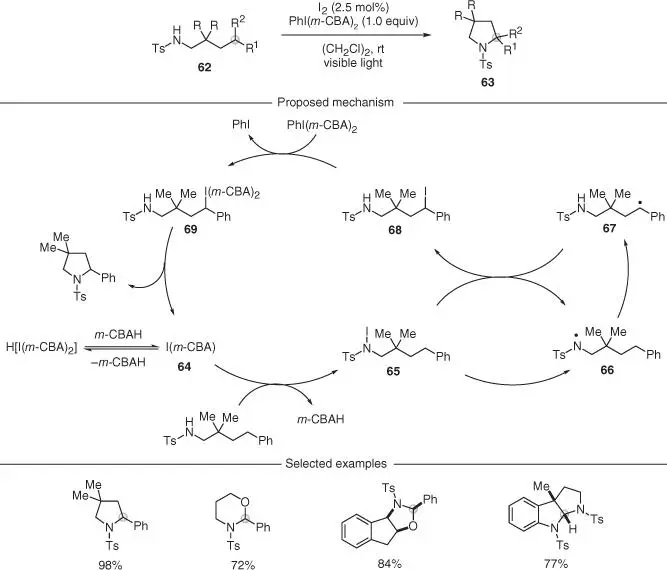
Scheme 2.16 Iodine‐catalyzed visible‐light mediated C–H aminations.
Source: Zhang and Muniz [44], Becker et al. [45], Martinez and Muniz [46].
More recently, this reactivity could also be triggered by catalytic generation of the corresponding N ‐bromo– N ‐Ts amine, which represents a catalytic version of the original HLF reactivity [47].
A different strategy based on N—I bond homolysis was recently introduced by Nagib and coworkers to generate imidate radicals, which could be used in HLF settings for C–H amination ( Scheme 2.17) [48]. This methodology started with simple aliphatic alcohols, which upon addition of Cl 3C–CN formed imidates 70. These species were iodinated by the in situ formation of I 2(KI + PhI(OAc) 2) and under visible light irradiation underwent N—I bond homolysis to generate the corresponding iminyl‐like nitrogen radicals. Upon 1,5‐HAT, the carbon radicals were iodinated, and then an ionic cyclization was used to access heterocycles, which, upon hydrolysis, gave the β ‐amino alcohol products 71.
The same authors later published a thermal, catalytic variant of the radical chaperone strategy, allowing them to generate analogous amino alcohols, however with less oxidant, which suppressed unwanted side reactions [49]. The side reactions occur from radicals derived from homolysis of excess AcOI, which can oxidize α‐aminyl C—H bonds, thereby competing with the desired 1,5‐HAT process.
Zhu and coworkers developed a protocol relying on the in situ formation of an N ‐iodo– N ‐Ts amine followed by photochemical N—I bond homolysis. While pioneering work by Gomez‐Suarez and coworkers [50–54] in the area focused on cyclization to form pyrrolidine derivatives, this new strategy sought to achieve an interrupted reactivity for selective sp 3C–H arylation of acyclic sulfonamides at distal positions ( Scheme 2.18). By taking simple reagents 72, in the presence of phenyl‐iodine bis(trifluoroacetate) (PIFA), they efficiently formed the corresponding N ‐iodo‐sulfonamide species. Upon visible light irradiation, N—I bond homolyses took place, furnishing the electrophilic sulfonamidyl radical. This species underwent efficient 1,5‐HAT, and the incipient carbon radical was intercepted with a protonated N‐heterocycle 73in a Minisci‐type reaction. Further oxidation delivered the desired γ‐arylated products 74in high yields. This methodology proved general with respect to the heteroaromatic coupling partner and could tolerate, for example, aryl halides, esters, and ethers. Furthermore, with respect to the amide portion, a number of different sulfonamides, carboxamides, and phosphoramides could be used, including a celecoxib derivative.
Читать дальше













