47 47 Paudyal, M.P., Adebesin, A.M., Burt, S.R. et al. (2016). Science 353: 1144.
48 48 Zhu, C., Li, G., Ess, D.H. et al. (2012). Journal of the American Chemical Society 134: 18253–18256.
49 49 Gao, H., Zhou, Z., Kwon, D.‐H. et al. (2017). Nature Chemistry 9: 681–688.
50 50 Behnke, N.E., Kielawa, R., Kwon, D.‐H. et al. (2018). Organic Letters 20: 8064–8068.
51 51 Farndon, J.J., Young, T.A., and Bower, J.F. (2018). Journal of the American Chemical Society 140: 17846–17850.
52 52 Zhou, Z., Cheng, Q.‐Q., and Kürti, L. (2019). Journal of the American Chemical Society 141: 2242–2246.
53 53 Cheng, Q.‐Q., Zhou, Z., Jiang, H. et al. (2020). Nature Catalysis 3: 386–392.
2 Remote Functionalizations Using Nitrogen Radicals in H‐Atom Transfer (HAT) Reactions
Ji Hye Kim, Elizabeth M. Dauncey, and Daniele Leonori
University of Manchester, Department of Chemistry, Oxford Road, Manchester, M13 9PL, UK
Nitrogen‐containing molecules represent a central class of compounds with applications spanning therapeutic agents, agrochemicals, food additives, and materials. Chemical methodologies able to streamline the synthesis and modification of these molecules underpin the development of high‐value products central to the well‐being of our society [1].
Nitrogen radicals are reactive intermediates with powerful applications to the synthesis of nitrogenated molecules [2]. Their reactivity can be used for direct C—N bond formation via exo‐trig cyclization ( Scheme 2.1a) and addition to electron‐rich π‐system ( Scheme 2.1b) or for remote sp 3carbon functionalization, exploiting their ability to partake in radical transpositions [3, 4]. From this perspective, there are two types of processes that can be used, β‐fission ( Scheme 2.1c) and intramolecular 1,5‐H‐atom transfer (1,5‐HAT) ( Scheme 2.1d). The former reactivity normally involves radical ring opening of small cyclic systems, while the latter enables the effective functionalization of N‐containing alkyl chains.
This chapter will discuss selected recent advances in the application of nitrogen radicals in the field of 1,5‐HAT. We will arrange the content highlighting the key mechanistic features of the HAT process and will then divide the discussion on the basis of the chemical process used to access the key nitrogen radicals. In each case, we aim to highlight the application of this radical reactivity in the assembly or the late‐stage functionalization of complex and bioactive materials.
2.2 Intramolecular 1,5‐H‐Atom Transfer (1,5‐HAT)
Nitrogen radicals are among the most efficient open‐shell intermediates known to undergo selective transpositions via intramolecular HAT [5, 6]. This reactivity can be used for the functionalization of nitrogenated alkyl chains at specific sp 3carbons that can be difficult to target by either ionic or transition‐metal‐mediated strategies [3]. The HAT process displays a strong 1,5‐selectivity, which means that the sp 3carbon δ to the nitrogen radical is the one that will be functionalized ( Scheme 2.2a). This high fidelity has played a fundamental role in the application of these processes in synthetic organic chemistry.
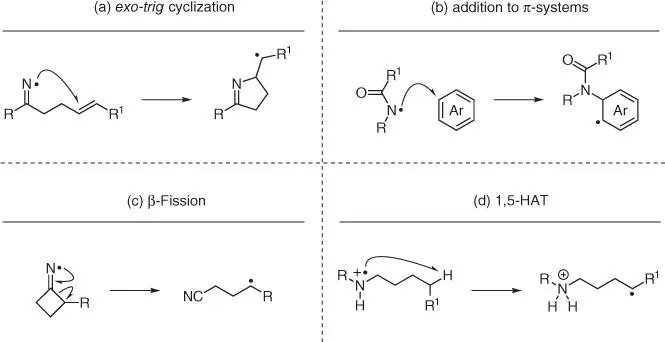
Scheme 2.1 Radical transposition processes using nitrogen radicals.
The ability of nitrogen radicals to undergo very efficient and effective transposition is also of high historical relevance to radical chemistry and organic synthesis as a whole. Indeed, this elementary step is the key event in the named reaction developed over 100 years ago by Hoffman, Löffler, and Freitag (HLF) [7]. Interestingly, the HLF reaction represents the first known example of radical transposition process and a very effective strategy for the construction of pyrrolidine heterocycles [5, 6]. In general terms, this reactivity is based on the conversion of a protonated N ‐haloamine ( 1) into an aminium radical ( 2) that undergoes very facile 1,5‐HAT, resulting in a nitrogen‐to‐carbon radical relay ( Scheme 2.2b). The incipient δ‐carbon radical ( 3) is then halogenated (S H2) ( 4) as part of an efficient radical chain propagation. A final basic treatment induces an ionic cyclization reaction, leading to the construction of a pyrrolidine scaffold ( 5). Although this reactivity has been established over 100 years ago, there is still continuous interest in further developments and innovations especially in terms of expanding the types of functionalizations and also control the overall stereochemical outcome.
Mechanistically, 1,5‐HAT reactions of nitrogen radicals are strongly polarity‐ and enthalpy‐driven processes with stringent geometrical constraints. This means that three main factors need to be considered when approaching the design of these transformations.
Geometrical factors. The 1,5‐HAT process goes through a six‐membered ring transition state, which, depending on the 3D structure of the nitrogen radical, might favor or thwart reactivity [8]. This is seen in a classical example of HLF reactivity for the assembly of a complex‐bridged azabicyclic system ( Scheme 2.3a) [9]. Although the intermediate N‐chloroamine 6 contains four different sites with 1,5‐relationship to the aminium radical 7, only the geometrically proximal methyl group is functionalized (8). Scheme 2.2 1,5‐HAT process in HLF reaction. Scheme 2.3 Geometrical factors, polar and enthalpic effects in 1,5‐HAT process.Source: (a) Katohgi et al. [9] and (c) Luo [10].
Polar effects. Nitrogen radicals are reactive intermediates with distinct and different philicities based on their substitution patterns. There are four general classes: (i) iminyl, (ii) amidyl (including carbamoyls, sulfamidyls, and phosphoramidyls), (iii) aminyl, and (iv) aminium radicals ( Scheme 2.3b). The philicity and the hybridization of these species change significantly: iminyl radicals [11] are σ‐radicals with ambiphilic character, while all the others are π‐radicals with the amidyls [12] and the aminiums [13] being electrophilic and the aminyls [14] slightly nucleophilic.
Although the 1,5‐HAT process goes via a sterically favorable six‐membered ring transition state, polar effects have a strong interplay in its stabilization [15]. This means that when the innate polarity of the H‐atom matches the one of the nitrogen radical, then a charge transfer stabilization takes place, leading to a more facile 1,5‐HAT process. As an example, the general amidyl radical 9 would undergo selective abstraction from the α‐O‐methylene group rather than the α‐ester one, as transition state 10 enables a better stabilization of polar effects than transition state 11.
Enthalpic effects. The exothermicity, hence the feasibility, of 1,5‐HAT reactions is dictated by the relative energy difference between the sp3 C—H bond broken and the N—H bond formed [16]. In general, N—H bonds have higher bond‐dissociation energy (BDEs) than sp3 C—H bonds, which facilitate 1,5‐HAT reactivities ( Scheme 2.3c) [10]. This is particularly evident for the reaction of amidyl and aminium radicals, which lead to the formation of very strong N—H bonds. Iminyl radicals have witnessed a more limited application in radical relay strategies owing to the weaker nature of their corresponding N—H bonds, which makes 1,5‐HAT possible mostly from activated or tertiary sp3 centers. Aminyl radicals are the only class of nitrogen radicals that are not used in these processes owing to their weaker N—H bonds and also to negative polar effects.
Читать дальше










