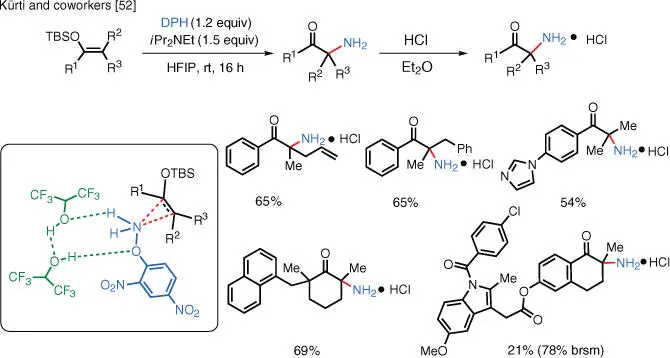
Scheme 1.36 TM‐free Rubottom oxidation.
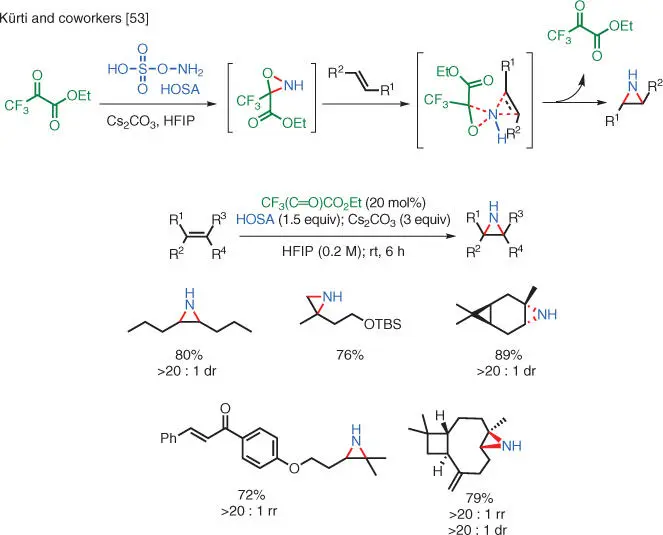
Scheme 1.37 TM‐free NH‐aziridination of unactivated olefins.
Source: Modified from Cheng et al. [53].
Two sets of conditions for the synthesis of primary α‐aminoketones from silyl enol ethers were developed. Electron‐rich substrates can be α‐aminated without transition metal catalysis, while substrates bearing electron‐withdrawing substituents can undergo α‐amination with the help of Rh or Cu catalysts.
Further studies in the Kürti group have shown that it is possible to achieve TM‐free NH‐aziridination of unactivated olefins with highly reactive NH‐oxaziridines via the “Butterfly Mechanism” [53]. These unique NH‐oxaziridines bear one or more strongly electron‐withdrawing group(s), which greatly enhance the electrophilicity of the nitrogen atom. With the further enhancement provided via the hydrogen bonding interactions by the HFIP solvent molecules, these highly reactive intermediates are capable of TM‐free transfer of the NH onto the unactivated olefins ( Scheme 1.37).
However, because of their high reactivities, these highly electron‐deficient NH‐oxaziridines cannot be isolated. The Kürti group solved this issue by forming these intermediates in situ from HOSA and ketones bearing electron‐withdrawing groups. Mass spectrometric studies confirmed the existence of the highly reactive oxaziridine intermediates.
By using a chiral nonracemic ketone as the organocatalyst, the reaction can give enantiomerically enriched products.
In the past two decades, a number of C—N bond‐forming reactions have been developed that take advantage of hydroxylamine‐based electrophilic aminating agents as sources of nitrogen. A great deal of structural diversity has been achieved in terms of the products. Olefins, substituted aromatic systems, as well as organometallic compounds have been successfully aminated. Although the vast majority of reported methods utilize transition metal complexes as catalysts, metal‐free and even organocatalytic methods have also emerged during the past decade. The two emerging trends are to incorporate unprotected amino groups directly and to use inexpensive and nontoxic transition metal catalysts such as iron complexes. We are confident that during the next decade, we will see further innovation in electrophilic amination chemistries.
1 1 (a) Canè, F., Brancaleoni, D., Dembech, P. et al. New developments on organocopper‐mediated electrophilic amination. In: New Horizons in Organic Synthesis, 118–129. New Age International Publishers.(b) Bernardi, P., Dembech, P., Ricci, A., and Seconi, G. (1999). The Journal of Organic Chemistry 64: 641–643, and references therein.(c) Corpet, M. and Gosmini, C. (2014). Synthesis‐Stuttgart 46: 2258–2271.
2 2 Erdik, E. and Ay, M. (1989). Chemical Reviews 89: 1947–1980.
3 3 Dong, X., Liu, Q., Dong, Y., and Liu, H. (2017). Chemistry (Easton). 23: 2481–2511.
4 4 Zhou, Z. and Kürti, L. (2019). Synlett 30: 1525–1535.
5 5 Tsutsui, H., Ichikawa, T., and Narasaka, K. (1999). Bulletin of the Chemical Society of Japan 72: 1869–1878.
6 6 Tsutsui, H., Hayashi, Y., and Narasaka, K. (1997). Chemistry Letters 26: 317–318.
7 7 Erdik, E. and Daşkapan, T. (1999). Synthetic Communications 29: 3989–3997.
8 8 Erdik, E. and Daşkapan, T. (1999). Journal of the Chemical Society, Perkin Transactions 1 21: 3139–3142.
9 9 Berman, A.M. and Johnson, J.S. (2004). Journal of the American Chemical Society 126: 5680–5681.
10 10 Berman, A.M. and Johnson, J.S. (2006). The Journal of Organic Chemistry 71: 219–224.
11 11 Garcia‐Lopez, J.A., Cetin, M., and Greaney, M.F. (2015). Angewandte Chemie (International Ed. in English) 54: 2156–2159.
12 12 Zhou, S., Yang, Z., Chen, X. et al. (2015). The Journal of Organic Chemistry 80: 6323–6328.
13 13 Nguyen, M.H. and Smith, A.B. (2013). Organic Letters 15: 4872–4875.
14 14 Zhou, Z., Ma, Z., Behnke, N.E. et al. (2017). Journal of the American Chemical Society 139: 115–118.
15 15 Tezuka, N., Shimojo, K., Hirano, K. et al. (2016). Journal of the American Chemical Society 138: 9166–9171.
16 16 Matsuda, N., Hirano, K., Satoh, T., and Miura, M. (2012). Angewandte Chemie (International Ed. in English) 51: 3642–3645.
17 17 Miki, Y., Hirano, K., Satoh, T., and Miura, M. (2013). Organic Letters 15: 172–175.
18 18 Miura, T., Morimoto, M., and Murakami, M. (2012). Organic Letters 14: 5214–5217.
19 19 Matsuda, N., Hirano, K., Satoh, T., and Miura, M. (2012). Angewandte Chemie (International Ed. in English) 51: 11827–11831.
20 20 Matsuda, N., Hirano, K., Satoh, T., and Miura, M. (2013). Journal of the American Chemical Society 135: 4934–4937.
21 21 Miki, Y., Hirano, K., Satoh, T., and Miura, M. (2013). Angewandte Chemie (International Ed. in English) 52: 10830–10834.
22 22 Zhu, S., Niljianskul, N., and Buchwald, S.L. (2013). Journal of the American Chemical Society 135: 15746–15749.
23 23 Shi, S.L. and Buchwald, S.L. (2015). Nature Chemistry 7: 38–44.
24 24 Matsuda, N., Hirano, K., Satoh, T., and Miura, M. (2012). The Journal of Organic Chemistry 77: 617–625.
25 25 Shen, K. and Wang, Q. (2015). Chemical Science 6: 4279–4283.
26 26 Hemric, B.N., Shen, K., and Wang, Q. (2016). Journal of the American Chemical Society 138: 5813–5816.
27 27 Ye, Z. and Dai, M. (2015). Organic Letters 17: 2190–2193.
28 28 Matsuda, N., Hirano, K., Satoh, T., and Miura, M. (2011). Organic Letters 13: 2860–2863.
29 29 Yotphan, S., Beukeaw, D., and Reutrakul, V. (2013). Tetrahedron 69: 6627–6633.
30 30 Zhu, C., Yi, M., Wei, D. et al. (2014). Organic Letters 16: 1840–1843.
31 31 Johnson, J.S. and Berman, A.M. (2005). Synlett 11: 1799–1801.
32 32 Barker, T.J. and Jarvo, E.R. (2009). Journal of the American Chemical Society 131: 15598–15599.
33 33 Lutter, F.H., Graßl, S., Grokenberger, L. et al. (2019). ChemCat Chem 11: 5188–5197.
34 34 Yoo, E.J., Ma, S., Mei, T.S. et al. (2011). Journal of the American Chemical Society 133: 7652–7655.
35 35 He, J., Shigenari, T., and Yu, J.Q. (2015). Angewandte Chemie International Edition 54: 6545–6549.
36 36 Shang, M., Zeng, S.‐H., Sun, S.‐Z. et al. (2013). Organic Letters 15: 5286–5289.
37 37 Dong, Z. and Dong, G. (2013). Journal of the American Chemical Society 135: 18350–18353.
38 38 Huehls, C.B., Lin, A., and Yang, J. (2014). Organic Letters 16: 3620–3623.
39 39 Dequirez, G., Pons, V., and Dauban, P. (2012). Angewandte Chemie (International Ed. in English) 51: 7384–7395.
40 40 Shimbayashi, T., Sasakura, K., Eguchi, A. et al. (2019). Chemistry (Easton). 25: 3156–3180.
41 41 Starkov, P., Jamison, T.F., and Marek, I. (2015). Chemistry (Easton). 21: 5278–5300.
42 42 Kwart, H. and Khan, A.A. (1967). Journal of the American Chemical Society 89: 1951–1953.
43 43 Jat, J.L., Paudyal, M.P., Gao, H. et al. (2014). Science 343: 61–65.
44 44 Nicolaou, K.C., Rhoades, D., Wang, Y. et al. (2017). Journal of the American Chemical Society 139: 7318–7334.
45 45 Ma, Z., Zhou, Z., and Kürti, L. (2017). Angewandte Chemie, International Edition 56: 9886–9890.
46 46 Strom, A.E. and Hartwig, J.F.J. (2013). Organic Chemistry 78: 8909–8914.
Читать дальше











