Following tooth eruption, interaction of the enamel surface with ions in the oral fluids leads to a small but significant increase in enamel maturation. This posteruptive maturation, especially if fluoride ion is present in the oral fluids, leads to additional improvement in the surface resistance of enamel to subsequent acid demineralization.
Structure of Postmaturation-Stage Ameloblasts
On completion of maturation, the maturation ameloblasts and the cells of the papillary layer undergo regression, reducing the quantity of their cytoplasmic organelles and their overall size. The postmaturation ameloblasts appear as low columnar cells, and the senescent papillary layer is reduced to one or two layers of low cuboidal cells. The reduced enamel epithelium remains in position, covering and protecting the enamel surface until the erupting tooth makes contact with the oral mucosa. At that stage, the reduced enamel epithelium fuses with the oral epithelium to form the primary junctional epithelium attachment to the cervical aspect of the crown.
Basic Science Correlations
Cytoplasmic organelles undergo rapid change during the many phases in the life cycle of the ameloblasts. These changes reflect the principal cellular functions that occur at each stage of amelogenesis. For example, the RER is most highly developed in secretory ameloblasts, lysosomes appear most abundant during the transition stage, and endocytotic coated vesicles are unusually prominent in papillary cells during maturation of enamel.
Significant changes in cell-to-cell contacts also occur throughout all phases of enamel formation. They appear to be needed for the coordination of cellular activity and for controlling the compartmentation of the extracellular space. These requirements are met by the formation of gap junctions and zonula occludens junctions. The following sections provide brief reviews of the structure and function of these two junctions.
Gap junctions
Gap junctions provide hydrophilic passageways across adjacent cell membranes for the intercellular exchange of ions and small molecules (less than 1,000 Da). 140, 141Special transmembrane gap junction proteins, called connexins ( Fig 3-12), create the channel through the membrane. The connexin molecule has four transmembrane domains, two rather rigid extracellular domains, and two cytoplasmic domains. 142The carboxy terminal domain, larger than the amino terminal domain, contains amino acid sequences that regulate channel permeability.
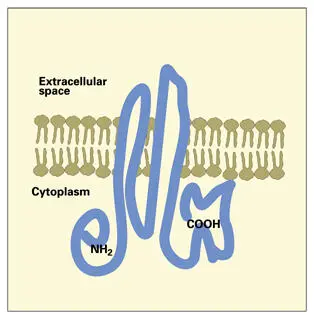
Fig 3-12Gap junction connexin protein from mammalian liver cells. The amino and carboxy terminals are located on the cytoplasmic surface. Two polypeptide loops of protein extend across the membrane to the external surface of the connexon.
Six connexin molecules aggregate in the membrane to form a supramolecular hemichannel, the connexon ( Fig 3-13). 143In forming a connexon hemichannel, the gap junction proteins assemble with their hydrophobic surfaces facing the lipid phase of the plasma membrane and their hydrophilic surfaces oriented inward (toward each other) to delimit a central fluid-filled channel across the membrane. When connexons from two adjacent cells are connected across the narrow intercellular gap, and the connexons are open, an intercytoplasmic exchange of ions and small molecules may occur. Typical gap junctions are made up of a hundred or more connexons aggregated in complementary patches in the cell membranes of a pair of participating cells ( Figs 3-13and 3-14).
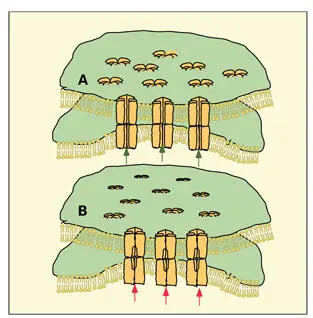
Fig 3-13Gap junction in the (A) coupled and (B) uncoupled states, showing the association of connexons in two juxtaposed plasma membranes. In the open condition (A), ions and small molecules can move through a fluid-filled pore (green arrows) from cell to cell. When the gap junction is uncoupled (B), the connexons are constricted and the pore is closed (red arrows) . (Adapted from Peracchia 143with permission from Kluwer Academic.)
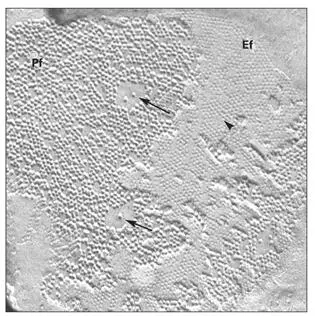
Fig 3-14Gap junction particles (arrows) are aggregated on the protoplasmic face (Pf) of the fractured plasma membrane. Pits (arrowhead) on the external face (Ef) of the membrane represent the position of the pore of the connexon particle. (Original magnification × 92,000.)
The flow of ions and small metabolites across gap junctions has been shown to be involved in coordinating and regulating cellular activity in groups of contiguous cells. For example, second messengers, such as cyclic adenosine monophosphate, calcium ions, and inositol triphosphate, have been shown to spread through gap junctions. 144In cardiac muscle, gap junctions, functioning as electrotonic synapses, coordinate the contraction of the heart. Gap junctions are needed during embryonic development to coordinate sequential differentiation of groups of cells.
Gap junction proteins are to some degree tissue specific. Lens, heart, and liver gap junction proteins have different molecular weights, suggesting that their respective connexons have tissue-specific physiologic functions in addition to common properties. The family of genes that encode connexins is made up of at least 12 members. 141, 145Homotypic and heterotypic assembly of connexin proteins result in gap junctions with different physiologic properties. 146In addition, it is possible for a single cell type to form different types of connexons and to restrict each type to specific domains of the cell membrane. 147
Participating cells are coupled when adjacent gap junction connexons are open. Various substances regulate the size of the pore opening and thereby control the degree of cell-to-cell coupling (reviewed by Bruzzone et al 148). Cytosolic calcium, cellular pH, retinoic acid, and intracellular oxygen tension have been shown to influence coupling. Connexons close within minutes in response to increased intracellular calcium, acidification of the cytoplasm, and low intracellular oxygen tension. This decoupling represents an emergency shutdown mechanism to prevent a cell-to-cell spread of noxious stimuli.
The calcium-binding protein, calmodulin, has been shown to participate in regulating the action of calcium on connexon proteins. Cyclic adenosine monophosphate modulates the number of gap junctions by increasing the rate of connexon assembly. Since gap junction proteins have a half-life of about 6 hours, there is a constant turnover of connexons at the cell surface.
Gap junctions are present between all cells of the enamel organ, suggesting that intercellular communication is necessary during all phases of enamel development. 11, 12, 96, 149, 150Immunocytochemical studies have shown that connexin 43 localizes in the SI, IEE, and preameloblasts. 151Information transferred across gap junctions may control cell proliferation and coordinate the activation and subsequent regulation of protein matrix secretion.
Large gap junctions are formed during enamel maturation. This may indicate that a bidirectional flow of ions from ameloblasts to papillary cells is a significant component of cellular activity during enamel maturation. Annular gap junctions are especially conspicuous in papillary cells. 150The latter are believed to represent stages in the internalization and breakdown of gap junctions.
Читать дальше