The negative end of the growing MT is stabilized by components of the MTOC. This arrangement permits the polarized growth of MTs away from the MTOC and toward the peripheral cytoplasm ( Fig 3-19). Microtubules radiate from the centrosome outward toward the plasma membrane, where the positive end of each MT is capped by special proteins.
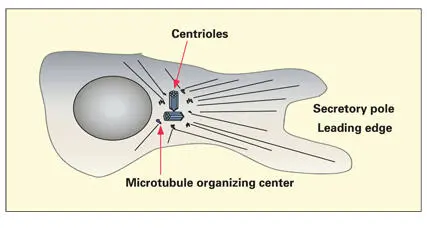
Fig 3-19Centrosome, consisting of a pair of centrioles and associated microtubule organizing centers. The centrosome regulates the polarization of the cellular microtubule network. The positive ends of the microtubules are located in the peripheral cytoplasm.
Microtubules are stabilized by interaction with capping proteins, microtubule-associated proteins, and by detyrosination (removal of tyrosine from the carboxy terminal of tubulin). Detyrosinated MTs constitute a small percentage of the total microtubular complement of the cell. They have a life span of about 2 hours. Most MTs are unstable. Unstable MTs are dynamic structures whose average life span is about 10 minutes.
Microtubules serve as conduits for the transport of organelles and vesicles. 157– 159Transport requires the action of microtubule-associated motor proteins (motor MAPs) and ATP. The most widely studied motor MAPs are the kinesin and dynein families of motor proteins. Classic kinesin is composed of two heavy chains and two light chains. 160The heavy chain contains a large N-terminal globular head group with binding sites for ATP and tubulin. The tail portions, stabilized in a helical conformation, contain binding sites for various integral membrane proteins that are contained in the limiting membranes of organelles, vesicles, and granules ( Fig 3-20). Dynein is a multimeric complex of heavy, intermediate, and light chains.
Motor MAPs transform the chemical energy released by the hydrolysis of ATP to adenosine diphosphate into mechanical displacement of the motor protein and its cargo along the surface of the MT (see Fig 3-20). It is unclear whether the movement of the motor protein and its cargo is caused by a conformational change (rachet power stroke) in the motor protein or by some form of biased diffusion along the MT surface. In general, kinesin transports cargo from the centrosome toward the peripheral cytoplasm, while dynein transports cargo in the opposite direction. For example, dynein has been shown to be needed for early endosome to late endosome and lysosomal transport, while kinesin is necessary for the transport of secretory granules from the trans-Golgi network to the plasma membrane.
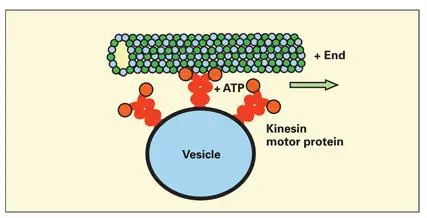
Fig 3-20Association of kinesin motor protein to a microtubule and the limiting membrane of a cytoplasmic vesicle. Adenosine triphosphatase (ATP) activity in the globular head group of the motor microtubule-associate protein causes displacement of the vesicle toward the positive end of the microtubule.
Individual members of these two families of motor MAPs appear to be relegated to the movement of specific cytoplasmic organelles and inclusions, for example, secretory granules, mitochondria, and transport vesicles. Specificity is believed to be a function of the binding affinity of carboxy tail domains of the motor MAP to target (receptor) proteins in the membrane of the transported entity.
Microtubule-associated structural proteins (structural MAPs) help to stabilize MTs by forming bridges to other cytoplasmic proteins. 156, 161They are present in high numbers in axons and dendrites of nerve cells. Approximately 15% to 20% of the total proteins of the brain are made of tubulin and MAPs.
Because of the abundance of structural MAPs in the brain, the neuronal MAPs have been most highly studied. Neuronal MAPs stabilize and promote the alignment of MTs in parallel arrays in axons and dendrites. In nonneuronal cells of the body, MTs are stabilized and bundled by MAP4. Phosphorylation of serine and threonine residues on MAPs by various kinases leads to MAP inactivation and decreased ability to interact with tubulin. On the other hand, the action of various phosphatases can activate MAPs and increase the organization of MT systems.
Clinical Correlations
Enamel dysplasia
Alteration of the ionic and metabolic milieu of secretory and maturation ameloblasts leads to defective enamel ( enamel dysplasia ). Because enamel does not remodel, its defects are retained in the fully formed tooth. Interference with the secretion stage leads to a reduction in the amount and/or composition of the enamel matrix, a condition known as enamel hypoplasia . The resulting enamel is thinner than normal, but usually fully mineralized. If the interference affects maturation ameloblasts, the result will be hypomaturation and hypomineralization. The resulting enamel contains more protein than normal, and the hydroxyapatite crystallites fail to reach their normal size.
Early maturation appears to be a critical stage in the formation of sound enamel, because disturbances occurring during the period when transitional ameloblasts differentiate into maturation ameloblasts lead to prolonged periods of suboptimal mineralization. 162Differentiating and secretory ameloblasts appear to have a greater potential for recovering normal function following metabolic insult.
Enamel affected by hypomaturation is usually of full thickness but more porous and less mineralized. Because it is less translucent, hypomaturated enamel appears clinically as white spots, or it may appear stained because of the subsequent absorption of extraneous molecules derived from food and serum. Dysplastic enamel usually contains physical evidence that both matrix deposition and maturation have been altered. Such teeth may have horizontal rows of pits and grooves of discolored and white opaque enamel.
Enamel dysplasia can be caused by local, systemic, and hereditary factors:
1. Anoxia in premature birth
2. Congenital syphilis
3. Erythrobastosis fetalis
4. Exantematous infections
5. Fluorosis
6. Hypoparathyroidism
7. Hypothyroidism
8. Renal hypophosphatasia
9. Vitamin A deficiency
10. Vitamin D–resistant rickets
In locally acting etiologies, there is no regular pattern involving contralateral teeth and no consistent pattern with relation to timing. An example of a local factor is an inflammatory process in a carious primary tooth that affects the nearby dental germ of its permanent replacement.
In a patient with enamel defects, symmetry of the lesions usually indicates a systemic cause. Systemic agents act in a symmetric and contemporaneous manner to involve all teeth under development at the time of the insult. Based on the position, distribution, and nature of the lesions, the approximate time period over which a disease occurred can be determined. The chronology of enamel formation ( Fig 3-21) reveals how a serious systemic disease, such as pneumonia or measles, affecting a 1-year-old child will cause enamel hypoplasia of the permanent incisors, canines, and first molars. 163A similar disease occurring in a 3-year-old child will affect the maturation phase in the incisors and canines and the secretory phase of the premolars. Recurrent systemic diseases, or the medications used in their treatment, frequently produce a series of symmetric horizontal ridges and grooves across the enamel surface.
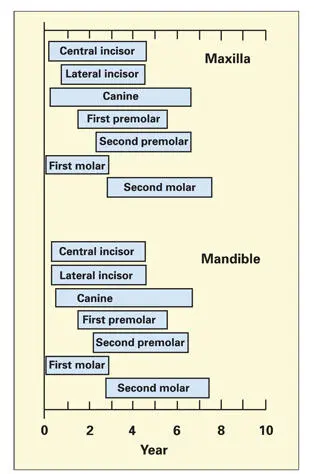
Fig 3-21Period of amelogenesis in the permanent teeth of the human dentition. Each bar represents the duration of enamel formation from beginning to completion of maturation. (Adapted from Seltzer and Bender. 163)
Читать дальше