Gap junction proteins have a rapid turnover time of approximately 5 hours. The functional significance of an apparent high turnover of gap junctions during the maturation phase remains to be explored.
Tight junctional complexes
Epithelial cells that are closely juxtaposed may participate in forming zones of fusion (tight junctions) between adjacent plasma membranes. For tight junctions to form, specific proteins must migrate from cytoplasmic pools to the cell surface to be inserted into the plasma membrane at points of cell-to-cell contact. Tight junctional contacts occur either as spotlike macula occludens, larger sheetlike fascia occludens, or as beltlike zonula occludens specializations.
The exact significance of the macula and fascia occludens junctions is unclear. Although these junctions cannot compartmentalize an extracellular space, they might provide increased cell-to-cell adhesion, or they might act as intramembrane stabilizers to restrict the lateral diffusion of other integral membrane proteins. In contrast, because the zonula occludens seals the extracellular space in a beltlike zone around the entire circumference of the cell, it compartmentalizes the extracellular space.
The zonula occludens plays two important functions in the physiology of epithelial layers. It provides a variable permeability barrier in the intercellular space, thereby creating isolated compartments and delineating luminal spaces. Second, by preventing lateral diffusion of integral proteins in the plasma membrane, it maintains specific domains in the cell membrane, such as the basolateral and apical surfaces of polarized cells. 152
The transmembrane protein responsible for creating the seal is called occludin . It binds additional proteins, zonula occludens 1 and zonula occludens 2, on its cytoplasmic domain. The zonula occludens proteins are kinases that may have signaling functions involved in regulating the degree of paracellular permeability. 153
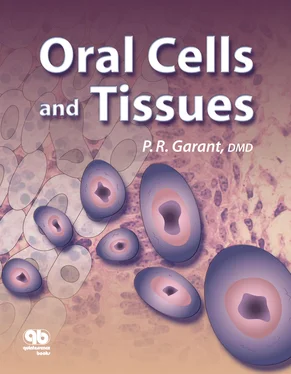
Structural analysis of the zonula occludens by electron microscopic observation of freeze-fracture replicas of the plasma membrane has shown that tight junctional proteins (occludin) assemble in linear strands or fibrils within the plasma membrane ( Figs 3-15aand 3-15b). The plasma membranes of the adjacent cells are fused along the linear strands of occludin proteins. The zonula occludens contains multiple anastomosing tight junctional strands.
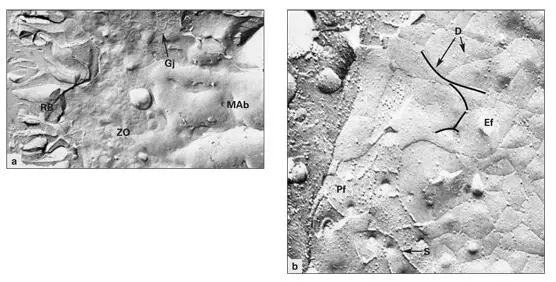
Figs 3-15a and 3-15bFreeze-fracture replica of the distal plasma membrane of a ruffle-ended maturation ameloblast (MAb). (a)Low magnification reveals the ruffled border (RB), components of the zonula occludens (ZO), and a gap junction (GJ). (Original magnification × 17,000.) (b)Higher magnification of the protoplasmic face (Pf). The tight junctional strands (S) of the zonula occludens are visible, as are depressions (D) created by the strands in the external face (Ef). (Original magnification × 80,000.)
Physiologic studies of epithelial permeability have shown that there is no clear correlation between the number and arrangement of tight junctional strands and the degree of intercellular occlusion. Some zonula occludens act as total barriers, while others (leaky tight junctions) permit the flow of ions and solutes through the paracellular space. Modulation of the contraction of the actomyosin ring (terminal web) associated with the zonula occludens and zonula adherens has been proposed as an explanation for differences in tight junctional permeability. Contraction of the actomyosin ring mediated by myosin light-chain kinase exerts tension on components of the zonula occludens, thereby altering the permeability of the paracellular space. 154
The presence of a zonula occludens at the distal end of the secretory ameloblast, just proximal to Tomes process, suggests that the space into which the enamel matrix is deposited is isolated from the intercellular spaces of the enamel organ. The enamel mineralization compartment is bounded below by mineralized dentin and above by the ameloblasts joined together by zonula occludens junctions. Analysis of the fluid contained in this compartment indicates that it has a different composition than the general extracellular fluid and serum.
In addition to creating an intercellular barrier, the zonula occludens of the secretory ameloblast may stabilize the secretory domain of Tomes process (analogous to the development of a luminal membrane compartment in other polarized secretory cells). The zonula occludens of the ruffle-ended ameloblast may have a similar role in maintaining the ruffled border and sealing the intercellular space of the enamel organ from the enamel compartment.
Microtubules and motor proteins in secretion
Microtubules (MTs) form a key component of the cytoskeleton in all cells. They provide a scaffold on which organelles, vesicles, and secretory granules are translocated by the action of motor proteins. In addition, MTs act as rigid struts involved in maintaining cell shape. During mitosis, MTs assemble to form the spindle apparatus required for chromosomal segregation.
Each MT is a hollow cylinder constructed of 13 protofilaments of tubulin. Tubulin protofilaments are assembled from heterodimers of α and β tubulin molecules ( Fig 3-16). 155The addition and removal of tubulin heterodimers takes place at opposite ends of an MT. The positive (+) end of an MT is the growing end, while the negative (–) end is the point of removal of tubulin. The removal of subunits at the negative end of an MT is slower than the rate of addition of new subunits at the positive end.
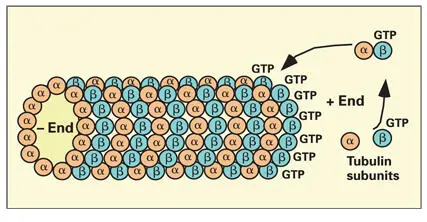
Fig 3-16Microtubule assembly by parallel association of tubulin protofilaments. Each protofilament forms by binding heterodimers of α and β tubulin at the positive end of the microtubule. Heterodimers are added when they are in the guanosine triphosphate (GTP)–bound state.
Both α and β tubulins are guanosine triphosphate (GTP)-binding proteins. In the GTP-bound state, the β tubulin subunit has a high binding affinity, thereby favoring rapid addition of subunits at the growing end of the elongating protofilament. 156Hydrolysis of GTP on the β tubulin subunits destabilizes the protofilament structure, causing rapid depolymerization of the MT ( Fig 3-17). Microtubules continue to grow as long as the rate of addition of GTP tubulin is faster than the rate of GTP hydrolysis.
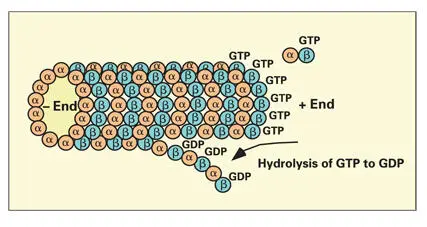
Fig 3-17Hydrolysis of guanosine triphosphate (GTP) to guanosine diphosphate (GDP). Hydrolysis of GTP on the β tubulin subunit destabilizes the protofilaments, leading to rapid depolymerization of microtubules.
Initiation of the polymerization of an MT requires the action of the microtubular organizing center (MTOC). The composition and mechanism of action of the MTOC is poorly understood. A third form of tubulin, γ tubulin, is found in MTOCs, where it performs a nucleating function. The most prominent MTOC is associated with the centrioles. Numerous MTOCs are located in the cytoplasm (the pericentriolar matrix) surrounding each pair of centrioles ( Fig 3-18).
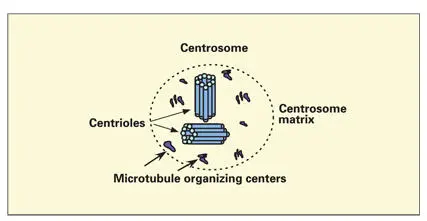
Fig 3-18Microtubule organizing centers. Numerous microtubule organizing centers are located in the centrosomal matrix associated with the centrioles. Each microtubule organizing center nucleates the development of a microtubule and stabilizes the microtubule by capping the negative end.
Читать дальше