It has been proposed that the intracellular transport of calcium could be carried out by several calcium-binding proteins localized in both secretory and maturation ameloblasts. 100– 102The recent identification of two low-affinity, high-capacity, calcium-binding proteins (calreticulin and endoplasmin) in the endoplasmic reticulum of secretory and maturation ameloblasts provides support for a new theory of calcium transcytosis involving the endoplasmic reticulum and inositol triphosphate–gated calcium channels. 103The endoplasmic reticulum could serve as a high-volume conduit for calcium transport across ameloblasts without altering the normal cytosolic calcium concentration. This theory would also explain why a large amount of endoplasmic reticulum but low levels of secretory protein synthesis are found in maturation ameloblasts. 103, 104
In situ hybridization with complementary deoxyribonucleic acid (cDNA) probes for bone sialoprotein is strongly positive in secretory ameloblasts. The potential role of bone sialoprotein, a calcium-binding protein common to most mineralized tissues, in enamel mineralization remains to be determined. 105It has been suggested that tuftelin and/or bone sialoprotein could trigger enamel crystal nucleation. 43, 73
Structure of Transition-Stage Ameloblasts
On completion of the full thickness of enamel, the secretory ameloblasts undergo cytoplasmic reorganization as they switch from a primarily protein secretory cell to that of an absorptive and transport cell. This process is characterized by extensive intracellular digestion of parts of the RER and other cytoplasmic organelles inside autophagosomes. During this stage, the ameloblasts contain high levels of acid phosphatase, indicative of increased lysosomal enzyme activity. The transition stage remodeling is so intensive that approximately 25% of the ameloblasts undergo programmed cell death. 106, 107
Surviving cells contain less RER, and their Golgi complexes contain many smooth vesicles and lysosomal-like structures. The development of a ruffled border, against the surface of the mineralized enamel, signifies the start of the rapid removal of water and protein from the enamel. At the completion of transition, the ameloblasts are shortened to half their previous height ( Figs 3-8aand 3-8b). They are now referred to as maturation ameloblasts .
Formation of the Papillary Layer
During the final phase of secretion, and progressing through the transition stage, the OEE, SR, and SI are transformed into the papillary layer, an epithelium believed to be specialized for transport. 11This conversion is preceded by a reduction in the size of the intercellular spaces of the SR and by a loss of glycosaminoglycans. The redifferentiated cells of the OEE, SR, and SI arrange themselves into numerous epithelial folds, or papillae, located between the ameloblasts and a well-developed capillary bed (see Figs 3-8aand 3-8b).
The former OEE, SR, and SI cells are no longer distinguishable as separate cell types, and are now referred to as papillary cells . Papillary cells contain numerous mitochondria, large numbers of pinocytotic vesicles, and extensive gap junctions. 108– 111Numerous microvilli increase the papillary cell surface area several fold.
The cytoplasmic features of the papillary cells, along with their association with a rich bed of fenestrated capillaries, suggest that at this stage the enamel organ has become specialized to perform transport functions related to enamel maturation. 112The fact that papillary cells form extensive gap junctions with adjacent maturation ameloblasts leads to the conclusion that these two types of cells are acting in concert during maturation. 11
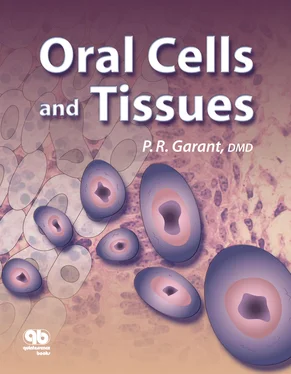
Figs 3-8a and 3-8bPapillary layer (PL) cells situated between the capillaries and the maturation ameloblasts (MA). (Hematoxylin-eosin stain. Original magnification × 600.) (a)Cross section depicting the MA through their long axis, and the alternating arrangement of papillae and indenting blood vessels. (E) Endothelial cells. (b)Tangential section through the maturation enamel organ, illustrating the close contact between papillary cells and capillaries filled with red blood cells (rbc).
Papillary cells have been shown to endocytose exogenous tracer material and to transport it to lysosomal bodies. 111This has led to the speculation that the papillary layer participates directly in the removal and degradation of enamel matrix breakdown products that gain access to the intercellular spaces of the papillary layer. However, there is no direct evidence that enamel matrix degradation products diffuse into the intercellular spaces of the papillary layer.
An alternative hypothesis suggests that sodium-potassium-ATPase activity in papillary cells generates an intercellular osmotic gradient across the enamel, drawing water and small matrix polypeptides toward the maturation ameloblasts. 113The polypeptide matrix fragments would undergo endocytosis and additional degradation in secondary lysosomes of maturation ameloblasts ( Figs 3-9to 3-11).
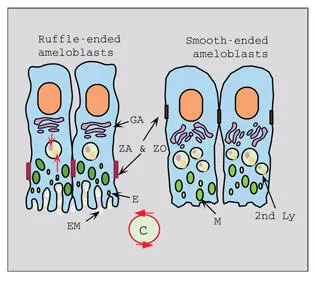
Fig 3-9Maturation ameloblast phenotypes. Ruffle-ended and smooth-ended maturation ameloblasts cycle back and forth during the maturation phase. Cycling (C) of the two phenotypes involves extensive remodeling of the distal cytoplasm and junctional complexes at both ends of the cells. The Golgi complexes (GA) and the lysosomal (Ly) apparatus are well developed in both cell configurations. Zonula adherens (ZA) and zonula occludens (ZO) shift from a distal position in the ruffle-ended ameloblasts to a proximal position in the smooth-ended ameloblasts. Mitochondria (M) are located primarily in the distal cytoplasm. Endosomes (E) containing enamel matrix (EM) are present in highest amount in the ruffle-ended ameloblasts.
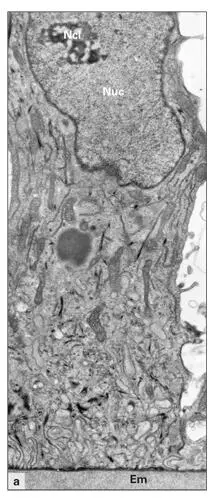
Fig 3-10aDistal part of a ruffle-ended maturation ameloblast. (Em) Enamel matrix; (Ncl) nucleolus; (Nuc) nucleus. (Original magnification × 9,000.)
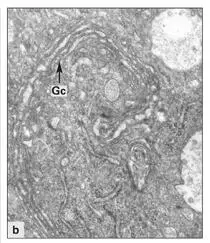
Fig 3-10bGolgi complex containing numerous Golgi cisternae (Gc) in a maturation ameloblast. (Original magnification × 16,000.)
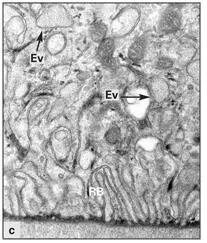
Fig 3-10cRuffled border (RB) and endocytosis vesicles (Ev) at higher magnification. (Original magnification × 20,000.)
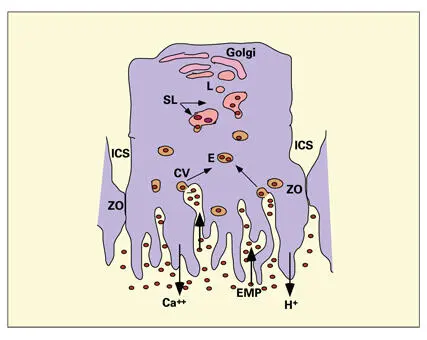
Fig 3-11Proposed pathway of enamel protein (EMP) reabsorption and digestion by ruffle-ended ameloblasts. The intercellular space (ICS) is sealed by a zonula occludens (ZO). Enamel proteins are endocytosed from the labyrinthine spaces of the ruffled border into endosomes (E) that fuse with larger secondary lysosomes (SL). Lysosomal enzymes are transported to the SLs via primary lysosomal vesicles (L) originating in the area of the Golgi apparatus. H +-adenosine triphosphatase, expressed at high levels, is responsible for secretion of H +into the enamel. High levels of alkaline phosphatase are correlated to calcium transport at the ruffled border. (CV) Coated vesicle.
Читать дальше