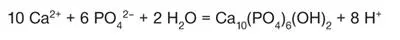Nonamelogenins
The nonamelogenin protein fraction contains relatively large (28- to 130-kDa) proteins of a generally acidic and hydrophilic nature. Several specific gene products have now been identified in the nonamelogenin fraction. These include tuftelin, ameloblastin, enamelin, and proteinases. Proteins of the nonamelogenin fraction demonstrate high binding affinity for hydroxyapatite crystals. 71These proteins are retained in small quantities in fully maturated enamel in the prism sheaths and as thin coatings surrounding the crystallites.
Tuftelin is a specific nonamelogenin acidic protein found in high concentration near the dentinoenamel junction and within enamel tufts. 21, 72Enamel tufts are hypomineralized developmental defects that extend perpendicularly from the dentinoenamel junction into the enamel. Tuftelin is the first enamel protein to be expressed during IEE differentiation. Tuftelin is a glycosylated protein with serine and threonine phosphorylation sites. Because of its composition, its early secretion, and its concentration at the mineralization front, tuftelin could have a role in nucleation of enamel crystallites. 73The human tuftelin gene has been localized on chromosome 1. 74
Ameloblastin, amelin, and sheathlin form a group of related “sheath” proteins that have been detected in rat, human, and porcine enamel. 16, 40, 75– 78Ameloblastin and sheathlin proteins accumulate between the plasma membrane of Tomes process and the growth zone of enamel prisms. 16, 79It has been suggested that sheath proteins may serve an early adhesive function in stabilizing the nonsecretory surface of TP to the enamel matrix. 77, 80
Soon after their secretion, the parent molecules undergo cleavage. The C-terminal polypeptide fragments are rapidly degraded and removed from the enamel. However, the N-terminal ameloblastin and sheathlin polypeptides are retained in prism sheaths. 79, 80Ameloblastin degradation products are less soluble than the parent molecule. 43Nonamelogenin protein fragments are believed to account for most of the remaining small percentage of protein contained in fully mature enamel.
Of additional interest is the report that amelin mRNA has been localized in preodontoblasts before it is expressed in ameloblasts. 78It has been suggested that amelin may have a role in the presecretory epithelial-mesenchymal interaction.
Enamelin is a high–molecular weight, acidic, glycosylated protein. It is secreted as a 186-kDa entity that subsequently undergoes progressive degradation to a 32-kDa molecule. 81The later fragment binds readily to enamel crystallites. 82The higher molecular weight fractions are localized along Tomes process and the newly developing enamel prism. The smaller fractions are located more deeply in the enamel, in association with the mineral in the prismatic and interprismatic domains. 81, 83
Some acidic proteins of the enamelin fraction are now known to be precursors of a group of serine proteinases that degrade enamel matrix. 58, 68, 69, 84
Despite recent progress in the biochemical characterization of the enamel matrix, knowledge of the sequential biochemical and biophysical interactions between mineral ions and enamel matrix proteins is still very incomplete. The most recently postulated roles for amelogenin and nonamelogenin proteins in initiating and controlling the construction of enamel have been reviewed by Nanci et al, 16Robinson et al, 43and Fincham et al. 51
Location and Expression of Amelogenin, Ameloblastin, and Tuftelin Genes
The gene for amelogenin ( AMEL ) has been mapped to the sex chromosomes ( Fig 3-7). In the rat, hamster, and mouse, Amel is present on the X chromosome 85; in humans, AMEL is present on both the X and Y chromosomes. 86, 87The gene on the Y chromosome ( AMELY ) is located in the q11 region, and the AMELX gene is located on the distal short arm (p22.1 to p22.3 positions) of the X chromosome. Recombination errors during the duplication of the sex chromosomes can lead to amelogenesis imperfecta.
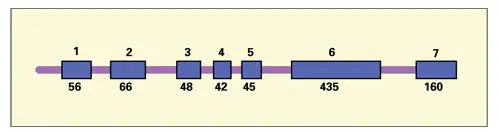
Fig 3-7Structure of the X-chromosomal copy of the human amelogenin gene. The bar segments represent the introns and the boxes (1 through 7) correspond to the exons. The nucleotide numbers are indicated below the exons. (Adapted from Simmer et al. 173)
The human amelogenin gene has seven introns and seven exons (see Fig 3-7). Both the X and Y amelogenin gene copies are expressed during tooth development. Transcription of the AMELX message appears several times more active than that of the Y copy, and the level of X-chromosomal amelogenin mRNA has been measured to be several fold higher than that of Y-chromosomal amelogenin mRNA.
A variety of amelogenin proteins are produced by alternative splicing of pre-mRNA. 46– 48Exons, and parts of exons, are deleted during alternative splicing. The resulting proteins all have a hydrophobic amino terminal, a large hydrophilic middle polypeptide, and a hydrophilic carboxy terminal. It is unclear if each amelogenin isoform performs a different function during enamel formation.
A small deletion in the AMELY gene permits it to be distinguished from its AMELX counterpart. This difference has proven useful in sex identification of human remains recovered from archeological sites 88and in forensic science. 89
The human tuftelin gene is located on chromosome 1. 74, 90The ameloblastin gene is localized to chromosome 4q21. 91
Mineralization of the Enamel Matrix
At the onset of enamel formation, the first enamel crystallites are spatially separated from the smaller dentin crystallites. High-resolution electron microscopy of the dentinoenamel junction indicates that the earliest enamel crystallites form from the alignment of dotlike mineral nuclei, approximately 2 to 4 nm in diameter. 92Chainlike association of these nuclei, apparently controlled by the amelogenin organic matrix, gives rise to long, needle-shaped crystallites. The crystallites develop in small clusters within extracellular deposits of amelogenin matrix, having the appearance of stippled material in electron micrographs.
Biochemical and electron probe analysis of the earliest crystallites suggests that the first mineral phase to be formed is a two-dimensional octacalciumphosphate precursor that subsequently transforms into hydroxyapatite. 66, 93The smallest hydroxyapatite crystal units (unit cells) are formed by the following reaction:
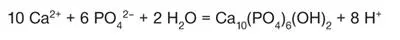
The hydrogen ions generated during crystal formation must be buffered to maintain a neutral pH to allow continued matrix mineralization. 44
Enamel crystal growth occurs in a compartment isolated between mineralized dentin and the zonula occludens junction of the ameloblastic layer. Elemental analysis indicates that the fluid in the mineralization compartment has a different composition than serum and extracellular fluid. 94, 95The presence of a distal zonula occludens junction between ameloblasts and the histochemical demonstration of calcium ATPase activity in the plasma membrane of Tomes process suggest that ameloblasts might control the fluid milieu within which enamel is deposited. 96– 98
Calcium ATPase has also been demonstrated in the distal cytoplasm of maturation ameloblasts. 98The recent localization of Ca 2+pump proteins in human secretory and early-stage maturation ameloblasts provides additional support for a functional plasma membrane calcium pump. 99The highest concentration of calcium pump protein was localized in the distal ends of ameloblasts near the mineralized enamel. 99
Читать дальше