Although the signal transduction pathways that regulate ameloblast and odontoblast differentiation have yet to be identified, immunohistochemical evidence has shown that cytokine-activated signaling pathways, including protein kinase C activation, are involved in early amelogenesis. 17
Structure of Secretion-Stage Ameloblasts
In describing the structure of the ameloblast, the term proximal is used to refer to the end of the cell nearest to the SI, and the term distal is used to identify the secretory end of the cell, next to the enamel (see Fig 3-2). The term apical is also used to describe the secretory pole of ameloblasts.
The structural changes that characterize each of the various stages of ameloblastic activity have been well documented. 18– 21The cytoplasm of the mature secretory ameloblast is highly polarized ( Figs 3-1to 3-4). The infranuclear (proximal) cytoplasm contains many mitochondria and a terminal web of cytoplasmic filaments associated with a zonula adherens (see Fig 3-2). Gap junctions are present between the proximal surface of the ameloblast and the overlying cell of the SI.
The supranuclear (distal) cytoplasm, which accounts for more than one half of the total cell volume, is filled with an extensive array of RER cisternae and a well-developed Golgi complex (see Figs 3-4ato 3-4c). 18, 20, 22Electron microscopic autoradiography and immunocytochemical studies have shown that enamel proteins are synthesized in the RER and glycosylated in the Golgi cisternae prior to being packaged into specific granules (see Figs 3-4ato 3-4c). 23– 25Condensing vacuoles derived from the trans-Golgi network mature into smaller, dense-core secretory granules. The newly formed secretory granules are immediately transported along a microtubular network to the distal end of the cell, where they are released by merocrine secretion into the enamel compartment. 26, 27Microtubule inhibitors, such as colchicine and vinblastine, block enamel matrix secretion. 28A distal junctional complex, consisting of gap junctions, a zonula adherens, and a zonula occludens, bind adjacent ameloblasts and seal the lateral intercellular spaces from the enamel-forming compartment (see Fig 3-3). 29
The first layer of enamel matrix (about 20 μm) is secreted across the flat distal cell surface of the newly differentiated ameloblast. As new membrane is added to the distal plasma membrane by the fusion of matrix secretion granules, the distal cell surface develops a protruding cytoplasmic process, 5 to 15 μm in length (see Figs 3-1, 3-3, and 3-4). Sir John Tomes, a British dentist and histologist, first described this process in the mid-19th century. The formation and the length of Tomes process (TP) appear to be related to the quantity and speed of matrix secretion, because new secretory granule membrane is added to the secretory pole of the cell faster than it can be recovered and recycled from that region. Tomes process protrudes at an angle to the long axis of the ameloblast cell body (see Fig 3-1).
With the formation of TP, the secretory surface of the ameloblast becomes more complex, and secretory granules are directed to two regions of the distal cytoplasm. 12, 26, 30Enamel matrix proteins released from the distoventral part of TP form prismatic or rod enamel (see Figs 3-3and 3-4). Secretion from the proximal part of TP, at the point where adjacent ameloblasts abut each other, gives rise to the interprismatic or interrod enamel (see Fig 3-2). The plasma membrane is highly infolded and apparently continuous with a tubulovesicular compartment at both the proximal and distal secretory sites. 25, 27The species-specific prism pattern is genetically determined by the shape and hexagonal packing of ameloblasts, the orientation of TP vis-à-vis the cell body, and the rate of enamel deposition. 31– 33
Observation of TP by electron microscopy has led to the conclusion that its surface can be subdivided into a secretory face (the concave or ventral surface) and a retrieval face (the convex or dorsolateral surface) (see Fig 3-3). 34Endocytosis for retrieval of membrane is carried out by formation of coated vesicles along the nonsecretory plasma membrane. Internalization and subsequent fusion of coated vesicles form endosomes and multivesicular bodies, components of the cell’s digestive apparatus. Studies of the fate of injected tracer substances have shown that they are taken up in coated vesicles of TP, suggesting that solutes in the extracellular space, including initial breakdown products of the enamel matrix, might begin to be removed in relatively small amounts during the secretory stage of amelogenesis.
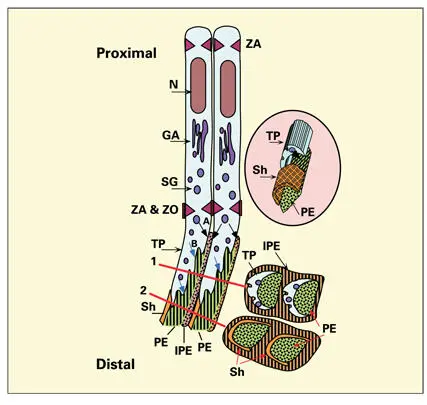
Fig 3-3Secretory ameloblasts. A zonula adherens (ZA) junction binds adjacent ameloblasts at both proximal and distal ends. The bulk of the infranuclear cytoplasm is occupied by the rough endoplasmic reticulum and a well-developed Golgi apparatus (GA). A zonula occludens (ZO) barrier is present in the intercellular space just proximal to Tomes process (TP). Secretion granules (SG), originating in the GA, are secreted from the proximal end of TP (A), giving rise to interprismatic enamel (IPE). Additional SG discharge at the distal end of the process (B) gives rise to a single enamel prism (PE). Interprismatic enamel is contributed by several contiguous cells. Cross sections of TP (1) and the adjacent enamel (2) illustrate the relationship of the secretory surface to prismatic enamel and the endocytotic surface to the development of a prism sheath (Sh). (N) Nucleus. (inset) Relationship among the prism sheath, the prism, and Tomes process.

Figs 3-4a to 3-4cElectron micrographs of secretory ameloblasts. (a)Distal portion of the ameloblasts, containing Golgi complexes (G) and an abundance of rough endoplasmic reticulum (RER). Secretion occurs from Tomes process (TP). (Nuc) Nucleus; (IPE) interprismatic enamel. (Original magnification × 2,200.) (b)High magnification of Tomes process (TP), containing secretion granules (SG). (IPE) Interprismatic enamel; (PE) prismatic enamel. (Original magnification × 17,000.) (c)Regular spacing of developing enamel crystallites (EnCR) in the enamel matrix (Em). (Original magnification × 94,000.)
During initial formation of enamel and during the last few days of enamel deposition, ameloblasts have no TP, and thus no prismatic pattern is formed; therefore, the first few microns of enamel next to dentin, and the last several microns of enamel at the surface, are aprismatic. The crystallites of aprismatic enamel are tightly packed and aligned perpendicular to the enamel surface. Aprismatic surface enamel compromises adhesion of dental occlusal sealants and orthodontic brackets by interfering with the penetration of adhesives into the enamel. 35This layer should be removed by acid etching before treatment protocols that require bonding to enamel.
Each ameloblast forms a single prism or enamel rod (see Fig 3-3). 12, 34, 36The enamel prism is made up of thousands of hydroxyapatite crystallites, oriented more or less parallel to each other. Each enamel crystallite is a ribbonlike structure that is believed to extend without interruption from the dentinoenamel junction to the enamel surface. 37Ultrastructural studies of enamel show that individual crystallites follow a spiral course within the prism. 26In longitudinal sections, enamel prisms exhibit optical cross striations, about 3.7 μm apart, caused by slight constrictions in the width of the prisms due to a daily cyclical rhythm of enamel matrix secretion. 38When human enamel is viewed in cross section, the prisms have an arcshaped outline and are arranged in offset horizontal rows (see Fig 3-3). 31
Читать дальше