Packing irregularities of crystallites demarcate the prismatic and interprismatic domains. This border region retains protein to form a sheathlike structure. Interprismatic crystallites have their long axes oriented at an angle to those in the prism (see Fig 3-3). The distinction between interprismatic and prismatic enamel is believed to reside solely in the orientation of crystallites. There is no evidence to suggest that the biochemical compositions of the interprismatic and prismatic matrices are different. Physicochemical forces, rather than biochemical differences in matrix proteins, act to orient the matrix and determine crystallite orientation at each of the two secretion sites. 26
A prism sheath ( Figs 3-3and 3-5) delimits approximately three quarters of the boundary between prismatic and interprismatic enamel. The composition of the sheath and its manner of development are not well understood. However, the shape of the sheath and its location over the convex surface of the prism suggest that its formation is associated with the endocytotic surface of Tomes process.
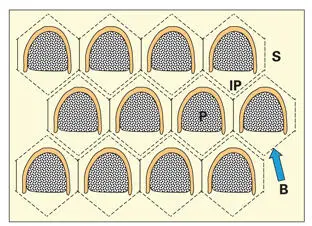
Fig 3-5Cross-sectional arrangement of the prisms in human enamel. The position of each ameloblast in relation to the prism outline is represented by the superimposed boundary lines (B). Each arcade-shaped prism is surrounded by interprismatic enamel (IP), which is contributed by the secretions of seven ameloblasts. Note the offset arrangement of the horizontal rows of arcades. (P) Prismatic enamel; (S) sheath region.
No sheath is present, and prismatic enamel is in direct contact with interprismatic enamel, along the flat surface of the prism, corresponding to the secretory surface of TP (see Fig 3-3). During enamel secretion, Tomes process undergoes fragmentation at its most distal point. This may create space between the already mineralized prismatic and aprismatic matrices. 39Several nonamelogenin proteins, collectively known as sheath proteins , appear to localize along TP, and in the space along prism boundaries. 40The space created along the prism boundary may provide a route for the escape of enamel matrix degradation products during enamel maturation. Retention of enamel protein fragments in the space created by the irregular packing of crystallites at the border between interprismatic enamel and prismatic enamel may contribute to the prism sheath.
Fully matured enamel provides a hard, wearresistant surface. Its only weakness, relative brittleness or susceptibility to crack formation, is along cleavage planes that follow the border between prismatic and interprismatic enamel. 41Biomechanical analysis of the fracture behavior of teeth has shown that the dentinoenamel junction undergoes plastic deformation to help resist crack propagation into the underlying dentin. 42Thus, most cracks are confined to enamel. It has been suggested that coarse collagen fibrils in the dentinoenamel junction resist and deflect crack propagation.
Biology of the Enamel Matrix
Information about the composition, mechanism of action in mineralization, and maturational change of enamel matrix proteins has been difficult to obtain because many enamel proteins are present in only relatively small amounts, and most undergo proteolytic processing soon after secretion. However, through the application of molecular biology techniques, significant progress is now being made in this area. 43Current understanding is that ameloblasts produce two classes of matrix proteins: amelogenin, a relatively homogenous product, which constitutes approximately 90% of newly secreted enamel matrix, and a heterogenous group of non-amelogenin proteins, including tuftelin, ameloblastin, enamelin, metalloproteinase, and serine proteinases, which make up the remaining 10%. The role of the enamel epithelium and the enamel matrix proteins in the mineralization of enamel has been the subject of several in-depth reviews. 43– 45
Amelogenins
Amelogenins are expressed as several isoforms through alternative splicing of pre–messenger ribonucleic acids (mRNAs). 12, 25, 30, 46– 49The amelogenins are rich in proline, leucine, glutamic acid, and histidine. Upon secretion the amelogenins form aggregates ( Fig 3-6). The hydrophilic carboxy terminals of the amelogenins are exposed at the surface of the aggregates, facing the water-mineral phase. The external anionic surface, containing phosphorylated serine, is believed to play a role in controlling crystal growth. 50, 51Following the cleavage of the hydrophilic terminals, the amelogenins self-assemble into supramolecular nanospheres approximately 18 nm in diameter (see Fig 3-6). 49– 54Each nanosphere comprises 100 to 200 amelogenin molecules stabilized by intermolecular hydrophobic interactions. High-resolution electron microscopy of newly secreted enamel matrix reveals nanospheres aligned between long, ribbonlike crystals of newly formed enamel. Presumably, adjacent crystallites are prevented from lateral fusion by the intervening amelogenin nanospheres, yet are able to grow rapidly along their C-axis. 49, 53, 55It is postulated that a scaffold of amelogenin nanospheres controls the orientation of the C-axis (long axis) of the developing hydroxyapatite crystals (see Figs 3-4and 3-6). 49, 53, 54, 56
Amelogenins are secreted as 25-kDa molecules that undergo progressive breakdown in the extracellular space. Proteases secreted by the enamel organ carry out specific and sequential proteolytic processing of the amelogenins. 57– 62The heavier amelogenins aggregate to form nanospheres that provide a structural scaffold to support the rapid and lengthwise growth of the crystallites (see Fig 3-6). 63– 66The smaller (20- and 13-kDa) fragments may slow crystallite growth in width and thickness by controlling the ionic activity of calcium in the enamel fluid. 64, 66, 67
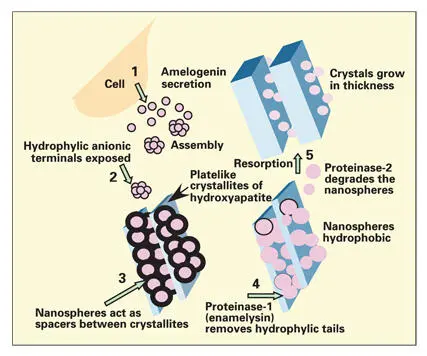
Fig 3-6Current concept of the role of amelogenins in the mineralization of enamel. The hydrophobic amelogenins form globular aggregates (nanospheres) on secretion into the extracellular space. The nanospheres form lattices that regulate the spacing and the orientation of the C-axis of the newly forming enamel crystallites. (Adapted from Fincham et al 51with permission from Elsevier Science.)
As the amelogenins complete their function, they are resorbed from the enamel matrix. Proteolytic enzymes from two classes of proteases, the serine proteases and the matrix metalloproteinases, appear responsible for degradation of enamel matrix (for review, see Woessner 60). Degradation of amelogenin is accomplished by specific proteinases produced by secretory and maturation ameloblasts. Cleavage of the hydrophilic carboxy terminal peptide by a serine protease initiates the degradation process. 68The rest of the amelogenin molecule is degraded by another serine proteinase (ameloprotease I), which appears to be a component of the enamelin fraction. 69
Recent studies suggest that metalloproteinases are involved in matrix degradation during the secretory-to-transition phase and that serine proteinases function mainly during the maturation phase. 43In situ hybridization and immunohistochemistry indicate that MMP-20 (enamelysin) is expressed in secretory ameloblasts and odontoblasts. 70Secretory ameloblasts may, to a limited extent, remove amelogenin peptides by endocytosis along the dorsal surfaces of Tomes process. However, the bulk of the amelogenin breakdown products are removed by maturation ameloblasts following the completion of the full thickness of the enamel layer.
Читать дальше