Fig 3-20Visual representation of layer separation following either the i-PRF (300g for 5 minutes) or C-PRF protocol (3000g for 8 minutes). Plasma was collected from the buffy coat region within the 1-mL layer above the RBC layer. (Reprinted with permission from Fujioka-Kobayashi et al. 11)
Optimization of C-PRF protocols
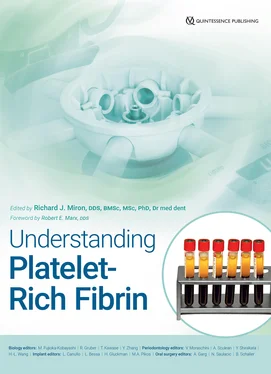
Prior to initiating the C-PRF experiments, protocols of 3000g for 5 minutes, 8 minutes, and 12 minutes were compared to optimize the accumulation of cells within the buffy coat layer. It was found that the 5-minute protocol was unable to concentrate all cells within the buffy coat layer, with the platelets remaining in the upper 4 mL. Following the 8-minute protocol, the sequential pipetting method results revealed that the majority of platelets and leukocytes were located within the buffy coat layer 6 region ( Fig 3-21). No further advantage was observed following the 12-minute protocol (data not shown). For comparison purposes, a standard i-PRF protocol resulted in a slight concentration of platelets and leukocytes in the upper 1-mL layer from which i-PRF was harvested (see Fig 3-21). Note that many platelets/leukocytes, however, remained in the RBC layers. To harvest C-PRF, a 1-mL layer within this buffy coat layer was collected and processed for further analysis ( Fig 3-22).
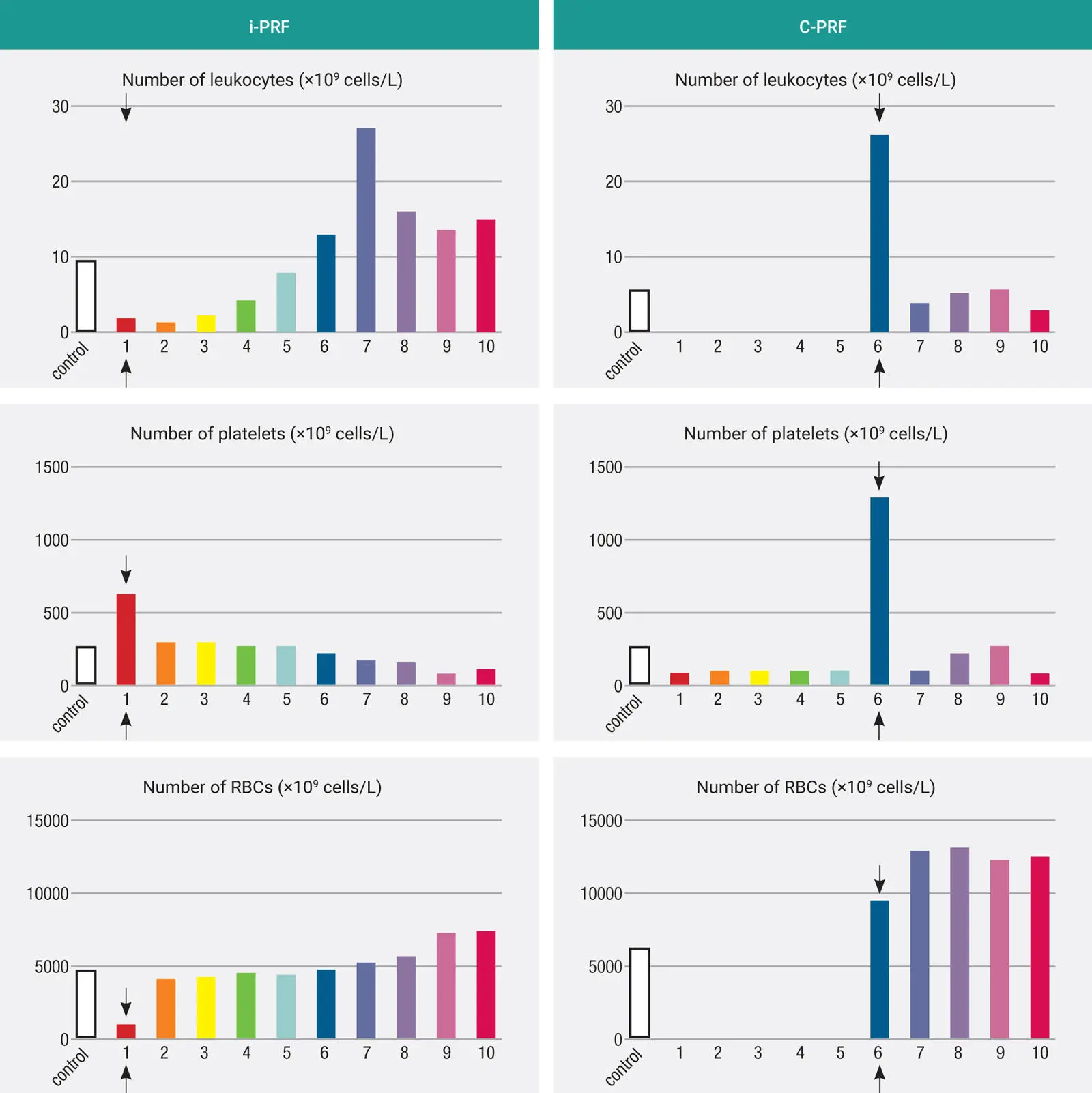
Fig 3-21The concentrations of different cell types found in each 1-mL layer of the 10-mL tube obtained through the i-PRF protocol (300g for 5 minutes) and the C-PRF protocol (3000g for 8 minutes). Note that in the PRF obtained through the i-PRF protocol, the majority of platelets and leukocytes were located in the 1-mL buffy coat layer. In the PRF obtained through the C-PRF protocol, although higher concentrations of platelets and leukocytes were found in the upper 1-mL layer, the majority of the platelets and leukocytes were actually located in the RBC layers. (Reprinted with permission from Fujioka-Kobayashi et al. 11)
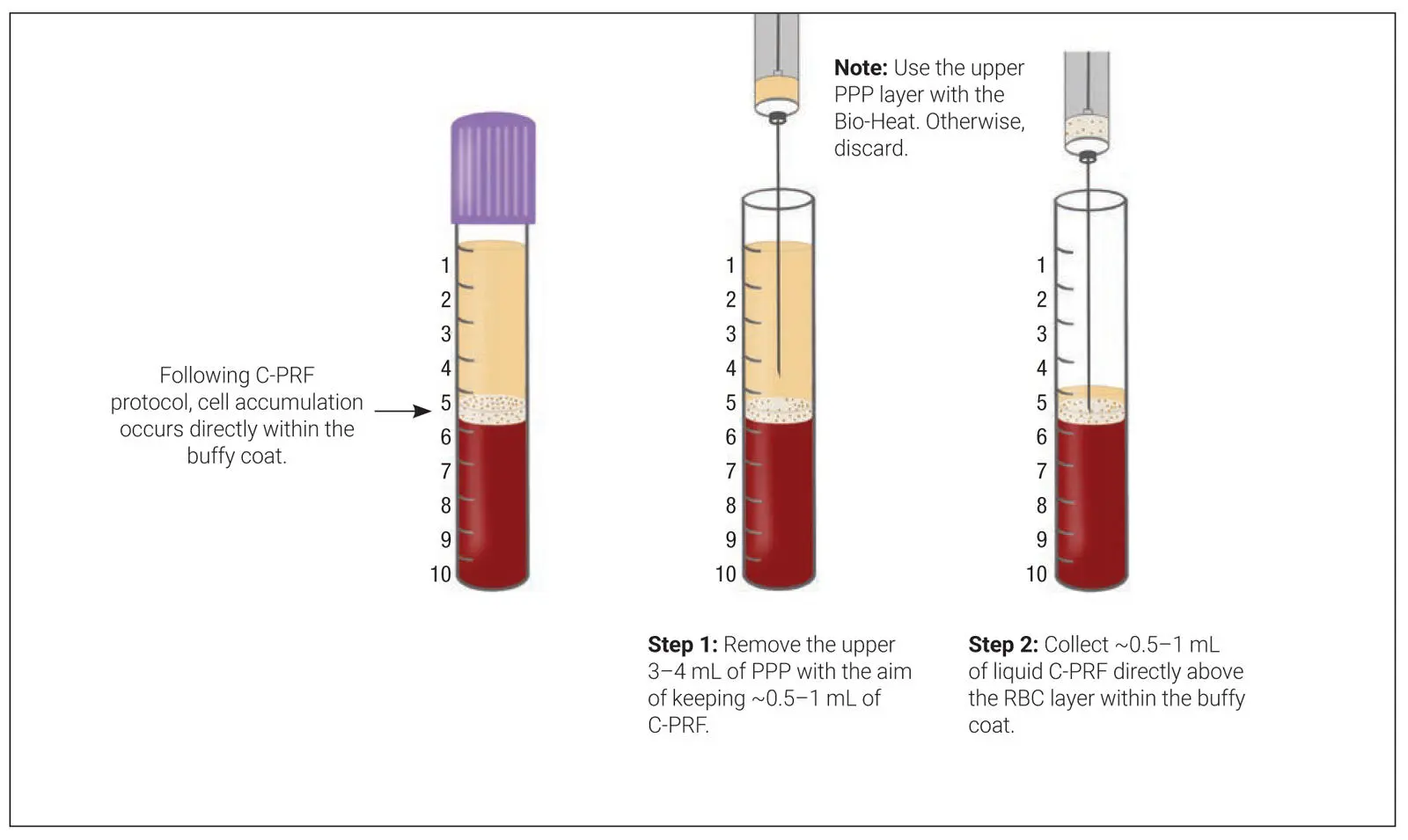
Fig 3-22Method to collect and concentrate C-PRF. Following centrifugation at higher speeds (2000g for 8 minutes), the majority of cells are located directly at the buffy coat layer. Instead of attempting to remove this layer with a long needle into the deep layers, it is highly advised to first remove the upper 4 mL of platelet-poor plasma (PPP), followed by collection of the C-PRF buffy coat layer.
Tips
In clinical practice, it is best to harvest C-PRF by first removing the upper 3 to 4 mL of platelet-poor plasma (discard it). The remaining C-PRF layer can then be taken much more easily. It is much more difficult to attempt to retrieve this buffy coat zone with 5 mL over top of it; it is harder to concentrate it, and too much volume is often collected.
Many centrifuges may not reach the 3000g speed. A 2000g protocol for 8 minutes will also achieve a C-PRF layer.
Comparative GF release between i-PRF and C-PRF
In the first set of experiments, the release of GFs including PDGF-AA, PDGF-AB, PDGF-BB, TGF-β1, VEGF, EGF, and IGF-1 from i-PRF and C-PRF was investigated by ELISA ( Fig 3-23). The release of all GFs over the entire 10-day (240-hour) period was significant for both protocols, with the C-PRF protocol resulting in up to two- to threefold higher quantities. This demonstrated clearly that this newly developed protocol had much greater regenerative potential when compared to previously utilized i-PRF protocols.
Fig 3-23Protein quantification of PDGF-AA (a) , PDGF-AB (b) , PDGF-BB (c) , TGF-β1 (d) , VEGF (e) , EGF (f) , and IGF-1 (g) at each time point over a 10-day (240-hour) period for PRF obtained through the i-PRF protocol and the C-PRF protocol, as determined by ELISA. Data represents the mean ± SE; an asterisk (*) indicates a value significantly higher than the other group ( P < .05). (Reprinted with permission from Fujioka-Kobayashi et al. 11)
Biocompatibility and cellular activity of i-PRF and C-PRF
It was first observed that while i-PRF induced a twofold increase in cell migration when compared to that observed in the control, a significantly higher fourfold increase was observed when cells were cultured with C-PRF ( Fig 3-24a). Furthermore, C-PRF also induced significantly higher cell proliferation at 3 and 5 days postseeding when compared to i-PRF ( Fig 3-24b). Both i-PRF and C-PRF were able to significantly upregulate TGF-β 3 days postseeding ( Fig 3-24c), and a significant 250% and 400% increase in the PDGF-AA was observed for i-PRF and C-PRF, respectively ( Fig 3-24d). The analysis of COL1 immunostaining also revealed significantly higher COL1A staining for C-PRF when compared to i-PRF and control tissue culture plastic groups ( Figs 3-24eand 3-24f).
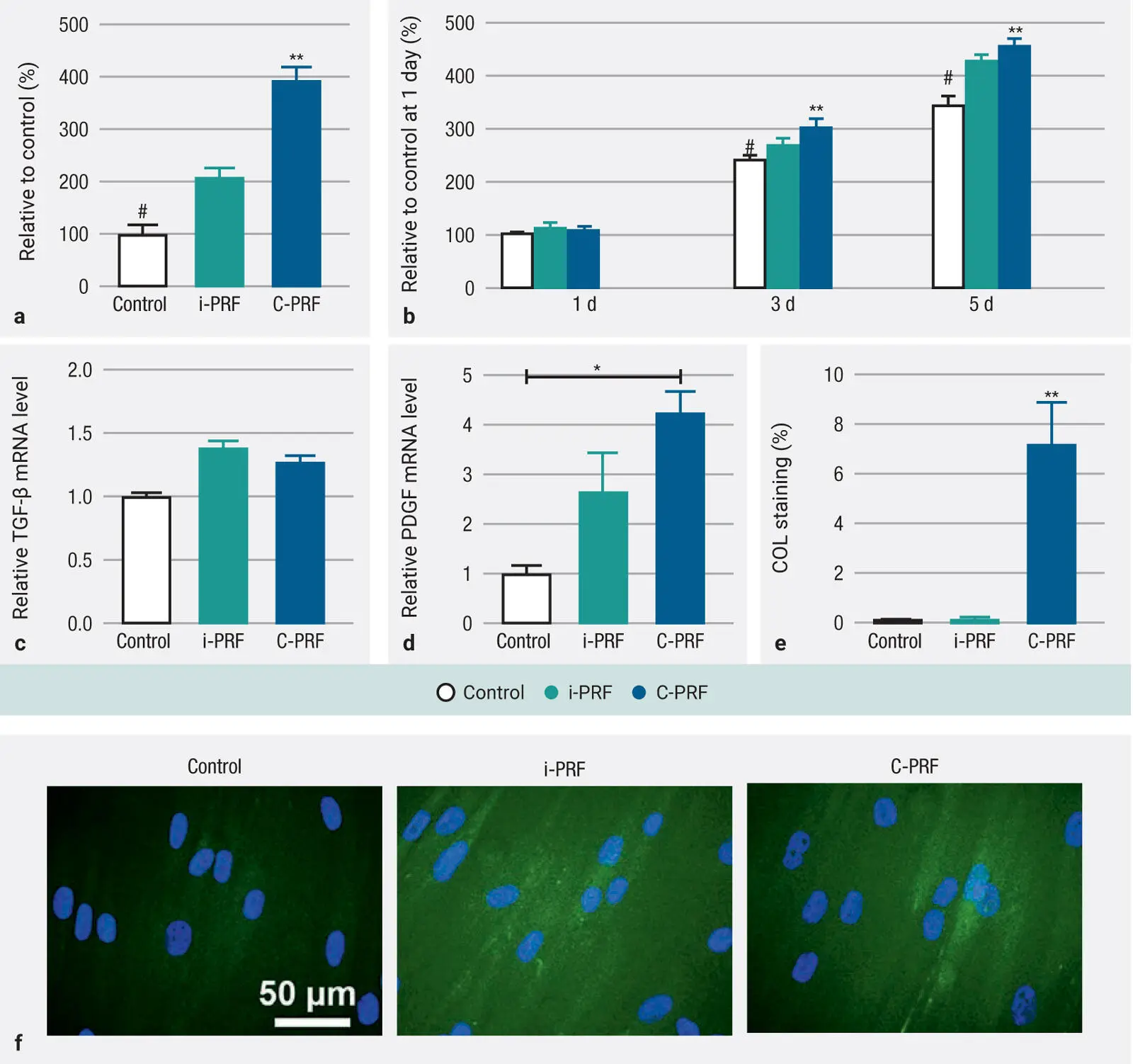
Fig 3-24 (a and b) Cell migration and proliferation at 1, 3, and 5 days in human gingival fibroblast (HGF-1) cells. (c and d) RT-PCR analysis of mRNA levels of TGF-β and PDGF in human gingival fibroblasts treated with i-PRF and C-PRF at 3 days. (e and f) Quantitative and representative staining of collagen1 at 14 days. Data represents mean ± SE; an asterisk (*) indicates a value significantly higher than the control group ( P < .05); a double asterisk (**) indicates a value significantly higher than all other groups ( P < .05); a number sign (#) indicates a value significantly lower than all groups ( P < .05). (Reprinted with permission from Fujioka-Kobayashi et al. 11)
Conclusion
In summary, the results from the 24-protocol investigation demonstrated clearly that certain protocols were better at increasing the amount of platelet/leukocyte yield (400g to 700g for 8 minutes), whereas others were more effective at concentrating PRF (200g to 300g for 5 minutes). Therefore, new protocols were designed accordingly for solid-PRF and liquid-PRF. While histologically it was observed that horizontal centrifugation led to better cell layer separation of blood cells as well as more even distribution of cells throughout the PRF clot, it was also histologically observed that the majority of cells accumulated on the back distal surfaces of PRF tubes when fixed-angle centrifugation was carried out. Lastly, a new C-PRF formulation was evaluated demonstrating up to a threefold increase in GF release during a 10-day period and further elicited fourfold increases in gingival fibroblast migration, PDGF gene expression, and collagen1 synthesis when compared to standard i-PRF protocols.
References
1.Miron RJ, Chai J, Zheng S, Feng M, Sculean A, Zhang Y. A novel method for evaluating and quantifying cell types in platelet rich fibrin and an introduction to horizontal centrifugation. J Biomed Mater Res A 2019;107:2257–2271.
2.Miron RJ, Dham A, Dham U, Zhang Y, Pikos MA, Sculean A. The effect of age, gender, and time between blood draw and start of centrifugation on the size outcomes of platelet-rich fibrin (PRF) membranes. Clin Oral Investig 2019;23:2179–2185.
Читать дальше